Fig. 1
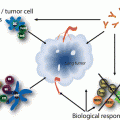
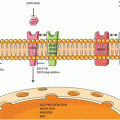
KRAS signaling. KRAS acts to integrate external signals from extracellular ligands that bind transmembrane growth factor receptors. The KRAS protein is localized to the cell membrane via farnesylation and shuttles between inactive and active states in response to these upstream signals. In its inactive state, KRAS is bound to GDP. Guanine nucleotide exchange factors (GEFs) catalyze the exchange of GDP for GTP, thereby activating KRAS. Activated KRAS results in increased downstream signaling of growth factor pathways involved in cellular proliferation, survival, and metastasis (e.g. RAF-MEK-ERK and PI3K-AKT-mTOR). GTPase-activating proteins (GAPs) hydrolyze GTP to GDP and result in signal termination
Three different RAS genes have been described in humans: KRAS, HRAS, and NRAS. The former two were first identified in the 1960s in studies of cancer-causing sarcoma viruses, namely the Kristen sarcoma virus and the Harvey sarcoma virus. NRAS was subsequently identified in human neuroblastoma cells. Activating point mutations in RAS lead to mutant RAS proteins that acquire transforming potential. This occurs secondary to impaired GTPase activity and constitutive activation of signaling.
Structurally, these mutations result in replacement of an amino acid at position 12, 13, or 61 [5]. The standard nomenclature used to describe these mutations denotes an amino acid change that occurs at one of these positions. For example, KRAS G12D refers to a point mutation resulting in the substitution of the amino acid glycine (G) that is found in the wild-type state at position 12 of the KRAS protein, with the amino acid asparate (D). Preclinical work in KRAS-mutant lung cancer cell lines has suggested that the type of amino acid substitution (e.g. G12C vs G12D) may affect downstream signaling differently, leading to a differential response to cytotoxic therapy [6]. Further studies are required to confirm this hypothesis.
KRAS-Mutant Lung Cancers: Clinicopathologic and Molecular Features
KRAS mutations account for the majority of RAS mutations in human malignancies. They are implicated in the pathogenesis of a variety of solid tumors. KRAS mutations have been identified in 60–90 % of pancreatic cancers, 35 % of colorectal cancers, 20 % of serous ovarian cancers, and 15 % of thyroid cancers [7].
KRAS mutations were first described in lung cancers in 1984 [8]. Santos et al. reported that an activating KRAS mutation was found in a human lung cancer specimen and not in normal tissue from the same patient, demonstrating that the mutation was somatically acquired. We have since come to learn that KRAS is the most commonly mutated oncogene in non-small cell lung carcinomas (NSCLCs), occurring with a frequency of approximately 25 % of unselected cases [9].
A variety of molecular diagnostics are used to identify mutations in KRAS. Testing can involve standard Sanger sequencing of the KRAS gene, or multiplex testing for specific mutational hotspots (e.g. Sequenom or SnapShot platforms). While these diagnostics have been extremely valuable over the last few decades, we are quickly moving into an era of massively-parallel high throughput or next-generation sequencing. In contrast to mutational hotspot testing that interrogates only specific mutations in KRAS, next generation sequencing affords the advantage of both the identification of mutations along the length of the gene and elucidation of aberrations in other tumor suppressor genes or oncogenes (Table 1).
Table 1
KRAS -mutant lung cancers. Methods used to diagnose KRAS mutations in non-small cell lung cancer specimens are listed here. In addition, the various clinicopathologic and molecular features associated with KRAS-mutant lung cancers are described
KRAS mutations in non-small cell lung cancers | |
|---|---|
Diagnosis | Traditional Sanger sequencing Multiplex mutational hotspot profiling Sequenom SnapShot Next-generation sequencing |
Clinical features | Classically associated with a significant history of current or former smoking While less common, can be found in a substantial proportion of never or former light smokers Reported to be more common in Caucasians compared to Asians |
Pathologic features | Found largely in lung adenocarcinomas Pathologic associations Solid growth pattern Mucinous tumors Tumor-infiltrating leukocytes |
Molecular features | Tend to be mutually exclusive with other lung cancer drivers (e.g. EGFR mutations, ALK fusions) Can coexist with various tumor suppressor gene aberrations Point mutation profile varies Transitions (G12D, G13D, G12S): more common in never or former light smokers Transversions (G12C, G12A, G12V, G13C): more common in patients with a significant smoking history |
KRAS mutations are largely found in lung adenocarcinomas although they have been infrequently described in squamous cell lung carcinomas where rigorous pathologic review was conducted [10, 11]. Rekhtman and colleagues tested 180 lung adenocarcinomas for mutations in KRAS and EGFR and assessed these specimens for the proportion of standard histologic patterns (acinar, lepidic, solid, mucinous, papillary, and micropapillary). In comparison to EGFR-mutant and KRAS/EGFR-wild-type tumors, the solid growth pattern was significantly over-represented in KRAS-mutant tumors. KRAS-mutations were also more commonly seen in mucinous adenocarcinomas that were significantly associated with the presence of tumor-infiltrating leukocytes [12].
Classically, KRAS mutations are thought to be found more commonly in patients with a significant history of smoking [13, 14]. In 2008, however, work by Riely et al. established that KRAS mutations are found in up to 15 % of never-smokers with lung cancers. We are now aware that this phenomenon extends to other drivers in lung cancers. While driver oncogenes can be found more commonly in patients with specific smoking histories (e.g. ALK fusions in never-smokers, BRAF mutations in current or former smokers), molecular testing should not be withheld from patients regardless of their pack-year history as these aberrations are identified in patients with varying degrees of exposure to tobacco. There is some evidence to support ethnic differences in the frequency of KRAS mutations, with a higher frequency noted in Caucasians compared to Asians and potentially African Americans [15, 16].
While exceptions to the rule have been reported, KRAS mutations tend to be mutually exclusive with other known lung cancer drivers such as EGFR mutations and ALK fusions. Substantial heterogeneity exists in the specific type of point mutation found in KRAS. In an analysis of data from the Catalogue of Somatic Mutations in Cancer (COSMIC), G12C was the most common mutation (42 %), followed by G12V (21 %), and G12D (17 %) [5]. A number of other mutations in codons 12, 13, and 61 have been described at lower frequencies.
Similar to what has been described with TP53, the type of point mutation found in KRAS-mutant lung cancers varies by smoking history. Transition point mutations (where a purine is exchanged for a purine or a pyrimidine for a pyrimidine, e.g. A→G or T→C, respectively), are more commonly found in never-smokers. The transition KRAS G12D is the most common point mutation in never-smokers. Other examples of transitions include G13D and G12S. Transversion point mutations (where a purine is exchanged for a pyrimidine or vice-versa, e.g. G →C or A→T), on the other hand, are more common in former/current smokers. KRAS G12C is the most common transversion in this population [17, 18]. Other examples of transversions include G12A, G12V, and G13C.
The Prognostic Nature of KRAS Mutations
Soon after the description of KRAS mutations in lung cancer in 1984, a number of studies began to emerge addressing the potential prognostic nature of these aberrations. In the 1990s, the prevailing sentiment was that KRAS mutations represented a negative prognostic marker for survival in patients with lung adenocarcinomas [19]. This viewpoint has since come into question as data from both individual and pooled studies have generated conflicting results.
Individual Studies
The role of KRAS mutations as a prognostic factor in lung cancer remains a controversial issue. While the prognostic nature of KRAS status has been studied widely in non-small cell lung carcinomas of all stages, patients with early-stage lung cancers represent a significant proportion of subjects. In comparison to studies in advanced-stage lung cancers, surgical specimens in early-stage studies afford the advantage of larger tumor samples on which molecular diagnostic testing can be more easily performed. These studies are summarized in Table 2.
Table 2
KRAS s tatus as a prognostic marker in lung cancer. Selected individual and pooled studies investigating the role of KRAS mutations as potential prognostic markers in non-small cell lung cancers are summarized here. While KRAS mutations were thought to be negative prognostic factors for survival, data remains conflicting
Reference | Patients tested for KRAS | Patients by KRAS status | Results KRAS-Mt vs KRAS WT | |
|---|---|---|---|---|
Mt | WT | |||
Selected individual studies | ||||
Kern et al. 1994 [20] | n = 44 Stage I–IV | 16 (36 %) | 28 (64 %) | HR for OS 1.7 (0.8–3.5), p = 0.16 |
Keohavong et al. 1996 [21] | n = 173 Stage I–IV | 43 (25 %) | 140 (75 %) | No difference in OS, p = 0.96 |
Graziano et al. 1999 [22] | n = 213 Stage I–II | 35 (16 %) | 178 (84 %) | Median OS 39 mo vs 53 mo, p = 0.33 |
Schiller et al. 2001 [9] | n = 184 Stage II–IIIA | 44 (24 %) | 140 (76 %) | Median OS 30 mo vs 42 mo, p = 0.38 |
Lu et al. 2004 [23] | n = 94 Stage I | 32 (34 %) | 62 (66 %) | HR for OS 1.18 (0.71–1.95), p = 0.52 |
Grossi et al. 2003 [24] | n = 249 Stage I–IIIA | 47 (19 %) | 202 (81 %) | HR for OS 1.46 (0.96–2.22), p = 0.08 |
Tsao et al. 2007 [25] | n = 450 Stage IB–II | 117 (26 %) | 333 (74 %) | HR for OS 1.23 (0.76–1.97), p = 0.40 |
Pooled analyses | ||||
Mascaux et al. meta-analysis, 2005 [26] | n = 3620 Stage I–IV | 18 % by PCR | 82 % by PCR | HR for OS 1.35 (1.16–1.56), p = 0.01 (KRAS mutation or p21 expression) |
Shepherd et al. LACE-Bio, 2013 [27] | n = 1,532 Stage I–III | 300 (19 %) | 1,232 (80 %) | HR for OS 1.17 (0.96–1.42), p = 0.12 |
The Eastern Cooperative Oncology Group (ECOG) E4592 study was a randomized control trial investigating the potential benefit of adjuvant thoracic radiation with or without four cycles of cisplatin/etoposide in patients with resected stage II-IIIA non-small cell lung cancers. This was the first large prospective study that assessed the role of KRAS mutations as determinants of prognosis. 184 tumors in this study were evaluated for KRAS status. Of the 44 KRAS-mutant tumors, 33 % were of non-squamous histology and 4.8 % were squamous cell carcinomas. While the overall survival of patients with KRAS-mutant lung cancers was numerically inferior to patients with KRAS wild-type lung cancers, this finding was not statistically significant. The median overall survival of patients with KRAS-mutant tumors was 30 months, compared to 42 months for patients with KRAS wild-type tumors (p = 0.38). On multivariate analysis, only age and tumor stage were found to be significant prognostic factors, although a trend was observed bordering on statistical significance for KRAS status (p = 0.07) [9].
In the JBR.10 study of patients with resected stage IB-II NSCLC, patients were randomized to receive either four cycles of adjuvant cisplatin/vinorelbine or observation. This was a positive trial that demonstrated a 15 % absolute improvement in 5-year survival in patients who received chemotherapy versus observation (HR, 0.78; 95 % CI, 0.61–0.99; p = 0.04). Overall, RAS mutations were detected in 24 % of patients, and this finding was not prognostic for overall survival (p = 0.40) [28].
The International Agency for Research on Cancer (IACR) examined the effect of KRAS status in early stage resected NSCLCs in the European Early Lung Cancer trial. KRAS mutations were identified in 18.5 % of tumors, 30.6 % of which were lung adenocarcinomas (n = 41/134) and 4.3 % squamous cell carcinomas (n = 5/115). KRAS status was not shown to be prognostic for progression-free survival (p = 0.26) [29].
The Cancer and Leukemia Group B-9633 (CALGB-9633) trial was a phase III study that randomized patients with stage IB NSCLC to either four cycles of adjuvant carboplatin/paclitaxel or observation. The study was a negative trial that failed to meet its primary endpoint. KRAS mutations were detected in 27 % of tumors (n = 71/258), and no prognostic effect of KRAS status was demonstrated (HR for OS 1.1, p = 0.747) [30].
Pooled Analyses
The individual studies discussed in the previous section have largely been limited by small numbers and insufficient power to draw conclusions on the prognostic nature of KRAS status in non-small cell lung cancers. Thus, a number of meta-analyses have been performed in attempt to answer this question.
Mascaux and colleagues published a meta-analysis of 28 studies and 3620 patients aimed at assessing the prognostic significance of KRAS status on both disease-free survival and overall survival in the adjuvant setting. The study demonstrated that the presence of a KRAS mutation was a negative prognostic factor for overall survival (HR for OS 1.35, CI 1.16–1.56, p = 0.01, random effect model), although this took into account both patients with a KRAS mutation and p21 expression. Similarly, KRAS mutations portended poorer overall survival in patients with lung adenocarcinomas (HR 1.52, CI 1.30–1.78, p = 0.02). These findings were not seen in the subset of patients with squamous cell lung carcinomas (HR 1.49, CI 0.88–2.52, p = 0.48). KRAS status was a significant prognostic marker when polymerase chain reaction sequencing was employed as the method of assessment (HR 1.39, CI 1.22–1.58, p = 0.03). In contrast, immunohistochemistry (IHC) to detect p21 status was not found to be significantly prognostic (1.08, CI 0.86–1.34, p = 0.21) [26].
Pooled data from 1721 patients from four randomized control trials comparing adjuvant chemotherapy versus observation (ANITA, IALT, JBR.10, and CALGB-9633) were analyzed as part of the LACE-Bio (Lung Adjuvant Cisplatin Evaluation-Biomarker) study. KRAS status was tested by restriction fragment length polymorphism, allelic specific oligonucleotide hybridization, or allelic refractory mutation system analysis and mass spectrometry. These detection methods were employed as they were deemed to demonstrate greater sensitivity than direct sequencing techniques [27].
KRAS status was successfully determined in 1532 patients. 20 % of these samples were found to be KRAS-mutant, of which 34 % were adenocarcinomas (n = 206/602), 6 % were squamous cell carcinomas (n = 44/705.) and 23 % were from non-squamous, non-adenocarcinoma histologies (n = 53/229.) KRAS mutations were found to be more frequent in women, younger patients, and early stage disease. In a multivariate analysis, only age (p = 0.04) and histology (p < 0.0001) were significant prognostic indicators.
There was no significant difference in overall survival based on KRAS status (HR for OS 1.17, CI 0.96–1.42, p = 0.12) with no heterogeneity among trials (p = 0.47). Similarly, there was no significant difference in disease-free survival based on KRAS status (HR for DFS 1.15, CI 0.96 = 1.39, p = 0.14). No prognostic difference for overall survival was demonstrated between different types of KRAS mutations, such as those involving codon 12 (HR: 1.04, CI 0.77–1.40) or codon 13 (HR 1.01, CI, 0.47–2.17, p = 0.96). There was no significant difference in prognosis for codon 12 subgroups for both disease-free (p = 0.98) and overall survival (p = 0.99).
The Predictive Nature of KRAS Mutations
Benefit of Adjuvant Chemotherapy in Early-Stage Lung Cancers
A number of randomized studies have investigated whether KRAS status might predict for response to adjuvant chemotherapy in non-small cell lung cancers. Similar to what we have seen in the previous section on the prognostic value of KRAS status, these trials have demonstrated discordant results. To date, KRAS status has not served as a valuable criterion to determine if patients with resected non-small cell lung cancers should receive adjuvant platinum-based chemotherapy.
In the JBR.10 study of adjuvant cisplatin and vinorelbine in resected stage IB-II non-small cell lung cancers, no statistically significant benefit of chemotherapy over observation was observed in patients with RAS wild-type tumors (median survival 7.8 vs 6.6 years, HR, 0.84, CI 0.63–1.12; p = 0.24). Similar results were obtained for patients with RAS-mutant lung cancers (median survival 9.7 vs 7.8 years, HR 0.82, CI 0.50–1.35, p = 0.44). Although the interaction term was non-significant for disease-specific survival, RAS wild-type patients appeared to derive more benefit from chemotherapy (HR 0.72, CI 0.51–1.02, p = 0.06) compared with RAS-mutant patients (HR, 1.07, CI 0.61–1.88, p = 0.82) [28].
In the CALGB 9633 study of adjuvant carboplatin and paclitaxel for resected stage IB non-small cell lung cancers, 5-year overall survival was not significantly different between KRAS-mutant and KRAS wild-type patients that received chemotherapy (55 % vs 62 %, HR 1.2, p = 0.58). Five-year overall survival was likewise not different between KRAS-mutant and KRAS wild-type patients who were randomized to observation (67 % vs 59 %, HR 1.1, p = 0.75). Adjuvant chemotherapy in patients with large tumors (>4 cm) was not significantly associated with benefit in patients with KRAS wild-type (HR 0.69, p = 0.18) or KRAS-mutant patients (HR 1.2, p = 0.55) [30].
Given the limitations of previous studies, investigators pooled data from three Lung Adjuvant Cisplatin Evaluation (LACE) platinum-based adjuvant chemotherapy trials to examine the role of KRAS status. The analysis revealed no significant effect of KRAS status on overall survival benefit from adjuvant chemotherapy over observation (KRAS wild-type tumors HR 0.89, CI 0.76–1.05, p = 0.15; KRAS-mutant tumors HR 1.05, CI 0.76–1.46, p = 0.77). Results were not different among trials (p = 0.52). Results were similar for disease-free survival (KRAS wild-type tumors HR 0.86, CI 0.74–1.00, p = 0.04; KRAS-mutant tumors HR 0.93, CI 0.68–1.27, p = 0.65) [27].
In terms of the different types of KRAS mutations, no benefit in overall survival was seen in patients with codon 12 mutations (HR 0.95, CI 0.67–1.35, p = 0.77). However, patients with codon 13 mutations had worse outcomes with adjuvant chemotherapy compared to patients who did not receive chemotherapy (HR for OS 5.78, CI 2.06–16.2, p < 0.01). A variable effect on overall survival was seen with codon 12 mutations: G12A or G12R (HR 0.66, p = 0.48), G12C or G12V (HR 0.94, p = 0.77) and G12D or G12S (HR 1.39, p = 0.48) but these differences were not significant (comparison of four HRs, including wild-type, p = 0.76). The authors concluded that KRAS status cannot be used to select patients with non-small cell lung cancer for adjuvant chemotherapy.
Benefit of Chemotherapy in Advanced Lung Cancers
Little is known regarding the role of KRAS mutations as predictors of response or resistance to cytotoxic chemotherapy. As with adjuvant chemotherapy, currently available data do not support the use of KRAS status as a means of selecting patients for systemic chemotherapy.
In the phase III TRIBUTE (Tarceva Responses in Conjunction with Paclitaxel and Carboplatin trial in advanced NSCLC) trial, first-line chemotherapy with carboplatin, paclitaxel, and erlotinib was compared to carboplatin, paclitaxel, and placebo. KRAS mutations were present in 21 % of tumor samples tested. In patients that received carboplatin and paclitaxel alone, response rate was not different between patients with KRAS-mutant and KRAS wild-type tumors (23 % vs 26 %). Time to progression and overall survival in the KRAS-mutant and KRAS wild-type cohorts that received chemotherapy alone were as follows: TTP 6 mo vs 5.4 mo, OS 13.5 mo vs 11.3 mo [31].
Small retrospective studies have been reported that did not reveal statistically significant benefits for palliative chemotherapy based on KRAS status in patients with advanced non-small cell lung cancers [32, 33]. In a retrospective study by Levy and colleagues of 16 patients with KRAS-mutant/EGFR-wild-type and 19 patients with KRAS/EGFR-wild-type non-small cell lung cancers who received first-line platinum-based pemetrexed-containing chemotherapy, overall response rate was not significantly different (56 % vs 36 %, p = 0.30). Within the limits of a small retrospective series and variable follow-up, median progression-free survival was improved in KRAS-mutant patients vs KRAS wild-type patients (10.3 vs 5.7 mo, p = 0.03). Overall survival was not reported [34]. These results remain hypothesis-generating.
Benefit of EGFR-Directed Targeted Therapy
The KRAS protein lies directly downstream of the epidermal growth factor receptor (EGFR). Due to its position in the signaling cascade, mutant KRAS is hypothesized to cause persistent pathway activation independent of EGFR signaling, thus conferring resistance to EGFR tyrosine kinase inhibition (TKI). While activating EGFR mutations are recognized as the strongest predictors of benefit from EGFR TKI use, a number of trials in advanced non-small cell lung cancers have asked the question of whether or not KRAS mutations are negative predictors of response to EGFR TKI therapy (Table 3).
Table 3
KRAS s tatus as a predictive marker of EGFR TKI benefit. Selected individual and pooled studies investigating the role of KRAS mutations as potential predictive markers of EGFR tyrosine kinase inhibition (TKI) with gefitinib or erlotinib in non-small cell lung cancers are summarized here. In general, KRAS mutations are thought to confer resistance to therapy
Study | Arm | Endpoint | KRAS-Mt | KRAS WT |
|---|---|---|---|---|
TRIBUTE Eberhard et al. [31] | Carboplatin, paclitaxel, and erlotinib | ORR | 8 % | 26 % |
TTP | 3.4 mo | 5.3 mo | ||
OS | 4.4 mo | 12.1 mo | ||
Hirsch et al. [35] | Erlotinib | ORR | 7 % | 19 % |
PFS | 3 mo | 3 mo | ||
OS | 12 mo | 11 mo | ||
Massarelli et al. [36] | Erlotinib or gefitinib | ORR | 0 % | 13 % |
TTP | 1.7 mo | 2.4 mo | ||
OS | 5.0 mo | 9.4 mo | ||
TRUST Schneider et al. [37] | Erlotinib | ORR | 0 % | 9 % |
BR.21 Zhu et al. [38] | Erlotinib | ORR | 5 % | 10 % |
INTEREST Doulliard et al. [39] | Gefitinib | ORR | 0 % | 10 % |
PFS | 1.4 mo | 2.6 mo | ||
OS | 7.8 mo | 7.5 mo | ||
Mao et al. pooled [14] | Erlotinib or gefitinib | RR | 3 % | 26 % |
The potential role for KRAS status as a predictive marker, was investigated in two phase III clinical trials examining single agent EGFR TKI versus best supportive care. The NCIC BR.21 trial compared erlotinib with placebo in patients with stage IIIB/IV non-small cell lung cancers who received one or two prior chemotherapy regimens. Overall survival was improved from 4.7 to 6.7 months in patients who were randomized to placebo versus erlotinib (p < 0.01). No significant difference in response rate was noted in patients who received erlotinib with KRAS-mutant vs KRAS wild-type tumors (5 % vs 10 %, p = 0.69). An interaction test did not demonstrate a significant difference in survival based on KRAS status (interaction p = 0.09) [38]. A similar analysis was undertaken for the ISEL study (Iressa Survival Evaluation in Lung Cancer) of gefinitinib vs placebo in second- and third-line patients with advanced non-small cell lung cancers. A KRAS mutation was detected in 7.9 % (n = 12) of 152 tumor samples. Due to the limited number of cases detected, no reliable conclusion could be drawn from the impact of KRAS status on the benefit of gefitinib versus best-supportive care [35]. A number of other studies examining the role of KRAS status as a predictive marker of benefit from EGFR TKI inhibition are summarized in Table 3.
The TRIBUTE trial supports the potential role for KRAS as a negative predictor of response to erlotinib plus chemotherapy versus chemotherapy alone. In patients with KRAS-mutant tumors, overall response rate was lower in patients who received erlotinib and chemotherapy versus those that received erlotinib alone (ORR 8 % vs 23 %). Patients with KRAS-mutant tumors who were treated with erlotinib and chemotherapy had a shorter median time to progression (TTP 3.4 mo, CI 1.5–6.3) and overall survival (OS 4.4 mo, CI 3.4–12.9) compared to patients who received chemotherapy alone (TTP 6 mo, CI 4.9–7.1; OS 13.5 mo, CI 11.1–15.9). Among patients with KRAS-mutant tumors, the hazard ratio of erlotinib plus chemotherapy versus chemotherapy alone was 2.1 (CI 1.1–3.8) for OS and 1.9 (CI 1.1–3.6) for TTP [31].
Two meta-analyses have assessed the association between KRAS status and response to EGFR TKI in non-small cell lung cancers. Linardou and colleagues pooled data from 17 non-small cell lung cancer trials, representing a total of 165 patients with KRAS mutations. In this analysis, KRAS mutations were significantly associated with an absence of response to EGFR tyrosine kinase inhibition (sensitivity 0.21, specificity 0.94, positive likelihood ratio 3.52, negative likelihood ratio 0.84). A pooled sensitivity analysis demonstrated that no response was seen in some KRAS wild-type tumors, leading the authors to believe that resistance to EGFR TKIs is unlikely to be solely mediated by KRAS mutation status (0.21; 95 % CI: 0.16–0.28) [40].
Data from 22 trials in non-small cell lung cancer was pooled for a meta-analysis by Mao and colleagues. 16 % of these patients (n = 231/1470) harbored tumors with mutant KRAS. The response rate to EGFR tyrosine kinase inhibition was 26 % in patients with KRAS wild-type tumors compared to 3 % for KRAS-mutant tumors. The pooled relative risk for response was 0.29 (95 % CI: 0.18–0.47; p < 0.01). This analysis was mirrored in both Asian and Caucasian patients, with a relative risk of 0.22 in Asians (95 % CI: 0.07–0.63; p = 0.01) and 0.31 in Caucasians (95 % CI: 0.17–0.54; p < 0.01) [14].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree