Fig. 1
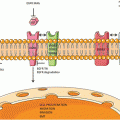
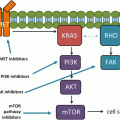
Ways to leave your EGFR inhibitor: biochemical pathways leading to resistance to small molecule EGFR drugs such as gefitinib and erlotinib. (a) Structures of two approved EGFR TKIs, gefitinib and erlotinib, used in the treatment of NSCLC. (b) Ribbon diagram of wild-type human EGFR (PDB code 2ITY), illustrating binding of gefitinib to the active site of the kinase. The magenta ball-stick (located just above the gefitinib molecule in the active site) indicates the gatekeeper residue (threonine790) that is commonly mutated to methionine (T790M), resulting in reduced inhibitor binding and drug resistance. (c) Simplified pathway diagram of EGFR signaling through RAS/MEK/ERK and PI3K/PDK1/AKT indicating the points of mutation/amplification in EGFR TKI resistance as reported by Sequist and colleagues. The resistance mechanisms include the EGFR T790M gatekeeper mutation, amplification of EGFR T790M, MET amplification, and PI3KCA mutation (note that additional epithelial to mesenchymal transition changes and transformation from the NSCLC to the SCLC phenotype also lead to resistance but are not covered by this illustration). The illustration also shows the FAS/NF-kB signaling arm downstream of the FAS death receptor that was shown to be important in TKI resistance by Bivona and colleagues (Reprinted from Cancer Cell, 19, Paul Workman and Paul A. Clarke, “Resisting Targeted Therapy: Fifty Ways to Leave Your EGFR”, 437–440, 2011, with permission from Elsevier)
Clinical and Surgical Implications of the EGFR-Mutations Pattern
1.
EGFR mutational profile in the pre-treatment assessment of NSCLC
As the EGFR mutational profile of NSCLCs is a strong predictor of response to therapy with the highly effective TKIs the most recent algorithms for the management of advanced NSCLCs underline the importance of EGFR molecular testing prior to the initiation of therapy and in particular, EGFR mutations should be sought in those NSCLCs in which they occur most frequently such us in the adenocarcinomas. Due to the recent understanding that histologic typing and EGFR mutation status are important for target the therapy in lung adenocarcinoma patients [2] there was a great need for a new classification that addresses diagnostic issues and strategic management to allow for molecular testing in small biopsy and cytology specimens.
All previous WHO classifications have addressed histologic classification primarily based on resection specimens. Since only 30 % of lung cancers are resectable, the vast majority of lung cancer patients present with advanced disease and are diagnosed based on small biopsy and cytology specimens. In 2011, the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society proposed a new classification for lung adenocarcinoma that included a number of changes to previous classifications. This classification now considers resection specimens and small biopsies well as cytology specimens. For resection specimens, the new terms of adenocarcinoma in situ and minimally invasive adenocarcinoma are introduced for small adenocarcinomas with pure lepidic and predominantly lepidic growth, with invasion ≤5 mm, respectively. Invasive adenocarcinomas are now classified by their predominant pattern as lepidic, acinar, papillary, and solid; a micropapillary pattern is newly added.
This classification also provides guidance for small biopsies due to the recent understanding that histologic typing and EGFR mutation status are important for target the therapy in lung adenocarcinoma patients. Actually, the value of adenocarcinoma in situ or minimally invasive adenocarcinoma for early stage NSCLC and the value of EGFR expressed in patients with advanced NSCLC predicting a benefit in terms of survival.
In the near future more surgical biopsies (in early and advanced disease) may be needed to define the best therapeutic strategy.
2.
EGFR mutational profile in post-treatment assessment of locally-advanced NSCLC
It seems that chemotherapy is able to modify the EGFR expression in NSCLCs by increasing or decreasing it [44].
This chemotherapy-related change may partially explain why chemotherapy resistant tumors are less sensitive to EGFR-TKI treatment than chemotherapy-naive tumors [45].
Moreover, the modification of the EGFR mutational pattern during chemotherapy may also explain why almost all clinical trials involving second-line TKI therapy have failed to show a positive correlation between EGFR mutation and progression-free or overall survival [26, 46].
Moreover, the shift in tumors from EGFR mutation status to wild-type status observed after first-line chemotherapy suggests that both mutant and nonmutant cancer cells coexist in the same tumor.
To identify intratumor heterogeneity, Bai and co-workers [47] microdissected and analyzed EGFR mutation status in more than 2506 tumor foci of 79 tumors from patients with NSCLC who underwent palliative surgery. Approximately 38 % of tumors contained both EGFR-mutant and wild-type foci. It is interesting to note that a majority of EGFRmutation changes after chemotherapy were from mutant state to wild type, suggesting that cancer cells harboring EGFR mutations might be more sensitive to chemotherapy than those without mutation. Furtherly, Bai and coll. analyzed the relationships between chemotherapy responses and the shift of EGFR mutation status and found patients who achieved PR were more likely to have had EGFR mutation shift than those achieving SD (Stable Disease) or PD (Progression Disease) after chemotherapy.
Therefore, it may be reasonable assumed that EGFR mutation shift could be related to the heterogeneity of intratumoral EGFR mutation and to different chemosensitivity levels of mutant and wild-type cells. These findings should be considered in future studies designed to elucidate the predictive role of EGFR mutation in second-line TKI therapy for patients with NSCLC.
Finally, no studies have been reported till now to investigate EGFR mutational pattern before and after chemotherapy administered with induction intent in locally-advanced potentially resectable (Stage IIIa) NSCLC. As the matter of fact, it may be interesting to investigate the effect of induction chemotherapy – usually based on cisplatinum derivates among with gemcitabine- on the EGFR mutational pattern, this representing an extreme simplified model first of all to evaluate the clonal resistance of neoplastic cells to drugs and also to investigate the biological response of the disease and the theoretical response to TKIs agents in alternative or in combination with surgical resection of the tumor following the induction protocol.
3.
EGFR mutational profile in the evaluation of suspicious second primary NSCLC
A better acknowledgement of the correlation between EGFR-mutations pattern and clonality in NSCLC may be extremely useful for other clinical scenarios.
As well, the assessment of multifocal lung tumours and the distinction of synchronous primary tumours from intrapulmonary metastases represents an important problem as this decision significantly influences tumour staging and subsequent treatment strategies.
In order to provide a basis for evidence-based treatment decisions in those patients, some Authors [48–50] have analysed the clonal relationship of multifocal NSCLC with indistinguishable histomorphology in a series of NSCLC patients.
In detail, Warth and co-workers have tested KRAS and EGFR mutations using polymorphic short tandem repeat markers in 78 suspicious multifocal NSCLC patients. Despite the limitation of the small sample, these preliminary data suggested a common clonal origin indicative of intrapulmonary metastases in almost two thirds (∼62 %) of the cases, while ∼36 % of multifocal NSCLC displayed unique molecular profiles suggesting separate primary tumours.
Therefore, as already suggested in 2 [51] although the IASLC/ATS/ERS classification recommended testing only patients with advanced adenocarcinomas for epidermal growth factor receptor (EGFR) mutations, we strongly advocate the assessment of EGFR mutations also in patients with synchronous/metachronous primary adenocarcinomas, because the eventual differences in clonality may indeed be a helpful tool for the differential diagnosis of pulmonary metastases vs secondary lung neoplasms.
Conclusion
It is reasonable to suggest that personalized therapy for NSCLC patients should include a genetic assessment of the EGFR mutational status for individual patients.
The appropriate role of an EGFR mutation routine analysis in the treatment of patients with NSCLC continues to evolve. In this context, preliminary evidences have emerged in the last decade, supporting the concept that EGFR mutation assessment may be a useful tool with clinically relevant implications in almost all settings of NSCLC treatment.
Further clinical trials should evaluate the ability of preoperative TKIs to achieve better results than can be obtained with platinum-based chemotherapy in locally advanced EGFRmut(+) NSCLC patients.
Finally, a close cooperation between clinicians, surgeons, molecular biologists and pathologists is crucial for a continuous improvement in the field of NSCLC target therapy.
References
1.
Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC (eds) (2004) World Health Organization classification of tumours. Pathology and genetics of tumours of the lung, pleura, thymus and heart. IARC Press, Lyon
2.
Antonicelli A, Cafarotti S, Indini A, Galli A et al (2013) EGFR-targeted therapy for non-small cell lung cancer: focus on EGFR oncogenic mutation. Int J Med Sci 10(3):320–330PubMedCentralCrossRefPubMed
3.
Travis WD et al (2011) IASLC/ATS/ERS international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 6(2):244–285PubMedCentralCrossRefPubMed
4.
5.
6.