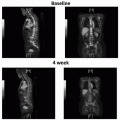
TNM Clinical Staging System |
Primary Tumor (T) |
Clinical |
|
TX |
Primary tumor cannot be assessed |
|
T0 |
No evidence of primary tumor |
|
T1 |
Clinically unapparent tumor neither palpable nor visible by imaging |
|
T1a |
Tumor incidental histologic finding in 5% or less of tissue resected |
|
T1b |
Tumor incidental histologic finding in more than 5% of tissue resected |
|
T1c |
Tumor identified by needle biopsy (e.g., because of elevated PSA) |
|
T2 |
Tumor confined within prostatea |
|
T2a |
Tumor involves one half of one lobe or less |
|
T2b |
Tumor involves more than one half of one lobe but not both lobes |
|
T2c |
Tumor involves both lobes |
|
T3 |
Tumor extends through the prostate capsuleb |
|
T3a |
Extracapsular extension (unilateral or bilateral) including microscopic bladder neck involvement |
|
T3b |
Tumor invades seminal vesicle(s) |
|
T4 |
Tumor is fixed or invades adjacent structures other than seminal vesicles: such as external sphincter, rectum, bladder, levator muscles, and/or pelvic wall |
Regional Lymph Nodes (N) |
Clinical |
|
NX |
Regional lymph nodes were not assessed |
|
N0 |
No regional lymph node metastasis |
|
N1 |
Metastasis in regional lymph node(s) |
Distant Metastasis (M) |
|
M0 |
No distant metastasis |
|
M1 |
Distant metastasis |
|
M1a |
Nonregional lymph node(s) |
|
M1b |
Bone(s) |
|
M1c |
Other site(s) with or without bone disease |
TNM Pathologic Staging System |
Primary Tumor (T)c |
Pathologic (pT) |
|
pT2 |
Organ confined |
|
pT2a |
Unilateral, one half of one side or less |
|
pT2b |
Unilateral, involving more than one half of side but not both sides |
|
pT2c |
Bilateral disease |
|
pT3 |
Extraprostatic extension |
|
pT3a |
Extraprostatic extension or microscopic invasion of bladder neckd |
|
pT3b |
Seminal vesicle invasion |
|
pT4 |
Invasion of rectum, levator muscles, and /or pelvic wall |
Regional Lymph Nodes (N) |
Pathologic |
|
pNX |
Regional nodes not sampled |
|
pN0 |
No positive regional nodes |
|
pN1 |
Metastases in regional node(s) |
Distant Metastasis (M) |
|
M0 |
No distant metastasis |
|
M1 |
Distant metastasis |
|
M1a |
Nonregional lymph node(s) |
|
M1b |
Bone(s) |
|
M1c |
Other site(s) with or without bone disease |
a There is no pathologic T1 classification.
b Positive surgical margin should be indicated by an R1 descriptor (residual microscopic disease).
c There is no pathologic T1 classification.
d Positive surgical margin should be indicated by an R1 descriptor (residual microscopic disease). |