BREAST CANCER DURING PREGNANCY
Pregnancy-associated breast cancer (PABC) or gestational breast cancer is defined as breast cancer that is diagnosed during pregnancy and the 12 months following delivery. Breast cancer is one of the most common cancers diagnosed during pregnancy. Given that many women are delaying childbearing (1), and age is a risk factor for breast cancer, the incidence of PABC appears to be on the rise. A study from the Swedish National Health Registry showed that the incidence of PABC increased from 16 to 37.4 per 100,000 deliveries between 1963 and 2002 (2). Factors associated with a diagnosis of breast cancer included age 35 years or older, women with private insurance, and women who delivered in an urban teaching hospital (3).
The most common clinical presentation of PABC is a painless mass that is either self-detected or noted on clinical exam. The duration of symptoms was significantly longer in patients with PABC compared to their nonpregnant counterparts in a study from Japan. Physiologic changes in a pregnant woman’s breast, especially in women younger than 30 years; physician familiarity with PABC; as well as socioeconomic and cultural factors may contribute to delays in diagnosis (4).
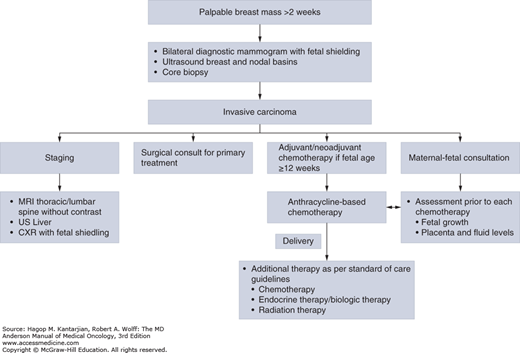
Although the majority of breast masses during pregnancy are benign, a breast mass that does not resolve within 2 weeks requires further investigation (2). Any clinically suspicious breast mass should be biopsied for a definitive diagnosis whether a patient is pregnant or not. Even though a number of small studies have shown the accuracy of fine-needle aspiration (FNA) in the diagnosis of PABC, a core or excisional biopsy of the breast lesion is necessary to make a diagnosis of invasion (5) (Fig. 30-1).
Two large surgical series of pregnant patients who had general anesthesia for a variety of underlying medical problems failed to demonstrate an increase in the risk of congenital malformations as compared with pregnant women who did not undergo surgery (6,7). Ultimately, the least-invasive and most technically accurate method(s) available should be utilized to determine the nature of a breast mass in a pregnant woman.
Mammography can be safely ordered during pregnancy with abdominal shielding. The estimated fetal radiation exposure was 0.4 mrad, well below the 5-rad threshold for fetal malformations (2). Ultrasound imaging is a preferred choice due to lack of radiation exposure and the better sensitivity for detecting breast masses in young patients. Ultrasound is also a valuable tool in evaluating nodal basins prior to treatment, as well as monitoring disease response in both in the breast and the nodal basins while on preoperative chemotherapy (8).
Magnetic resonance imaging (MRI) has not been studied for the diagnosis of breast masses in pregnant or lactating women. Gadolinium has been shown to cross the placenta and cause fetal abnormalities in animal studies. Given these safety concerns, Gadolinium is a Food and Drug Administration class C drug in pregnancy and should not be used routinely in the evaluation of breast masses in pregnancy (2).
The most common pathology of PABC is invasive ductal carcinoma. In an MD Anderson Cancer Center (MDACC) case series of patients with breast cancer treated with chemotherapy during pregnancy, PABC was diagnosed at a more advanced stage, with lymph node involvement and poor histologic and prognostic features, including high Ki67 and estrogen receptor (ER) and progesterone receptor (PR) negativity. HER2–positive tumors comprised 28% of the specimens. The most common pathology was infiltrating ductal carcinoma. The authors concluded these features were similar to those reported in young nonpregnant women, and that age rather than pregnancy may be the determinant of tumor biology (9).
The American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) system is used for staging in pregnant patients with breast cancer. Given that stage of diagnosis may have significant psychosocial implications, accurate staging is important.
A complete history and physical examination and laboratory work, including complete blood cell counts and metabolic panel, should be done prior to initiation of treatment. Local imaging should include mammography and ultrasound of the breast and the draining lymphatics. Given that women with PABC often present with advanced-stage disease, the major sites of metastatic disease (lung, liver, and bone) should be evaluated in patients with stage II or higher cancers. Evaluation includes chest radiography with abdominal shielding, ultrasound of the liver, and MRI of the spine without contrast. A transthoracic echocardiogram prior to initiation of anthracycline chemotherapy is recommended (2).
The goal of treatment in both pregnant and nonpregnant patients is the same: the control of local and systemic disease. Although the treatment strategies for pregnant and nonpregnant patients are similar, the impact of the treatment on the fetus and the outcome of the pregnancy should be considered in the pregnant patient with breast cancer.
As previously discussed, the use of anesthesia during pregnancy is not associated with an increased risk of fetal malformation (2). Most patients and surgeons elect to wait until the end of the first trimester to operate to minimize the risk of spontaneous abortion. In most reports of pregnant patients with breast cancer, the majority of women underwent modified radical mastectomy with axillary lymph node dissection, possibly reflecting treatment practice during that time period, later stage of diagnosis, or concerns over the need for radiation if breast-conserving surgery is performed. In general, breast radiation is contraindicated during pregnancy because of the risk of radiation exposure to the fetus. With new practices recommending systemic therapy and surgery as initial treatment strategies, radiation therapy can usually be delayed until after delivery (2,10).
In a recent series from MDACC, Dominici et al reported on 67 women diagnosed with PABC who received systemic chemotherapy during pregnancy and then proceeded to surgical management. Forty-seven patients underwent mastectomy, and 20 underwent breast-conserving surgery. There were a total of six postoperative complications, with all treated as outpatients; four complications were in the patients with mastectomy, and two were in the patients with breast-conserving surgery. The authors concluded breast-conserving surgery is feasible with no increase in the rate of complications (2).
Cardonick et al reported on 130 patients diagnosed with PABC from the Cancer and Pregnancy Registry. Ninety-five patients underwent surgery, 38 in the first, 48 in the second, and 9 in the third trimester. Fifty-four patients underwent mastectomy, 34 had a lumpectomy, and 15 had an excisional biopsy that did not require further surgery. There was no increase in the miscarriage rate in the first trimester (2).
There are limited data on the use of sentinel lymph node biopsy in pregnant patients with breast cancer. Isosulfane blue dye is not recommended due to reports of anaphylaxis in the patients as well as concerns for the safety of the fetus (2).
The indications for systemic therapy in a pregnant patient with breast cancer patient are similar to those in the nonpregnant patient. Most of the chemotherapy agents used in pregnancy are rated category D. Limited data are available on pharmacokinetics of antineoplastic agents in the setting of physiologic changes of pregnancy. In a review of 289 pregnant cancer patients treated with chemotherapy for a variety of malignancies, the 14% to 19% incidence of fetal malformations with first-trimester exposure dropped to 1.3% with exposure in the second and third trimesters. Cardonick et al reported a rate of congenital malformations of 3.8% in their series of 104 women who received chemotherapy during pregnancy (2).
The only published prospective cohort of pregnant patients with breast cancer treated with systemic chemotherapy during the second and third trimesters of pregnancy did not report any congenital malformations, stillbirths, or spontaneous abortions (11). The 57 women in this prospective series were treated with chemotherapy with FAC (5-fluorouracil 500 mg/m2 IV on days 1 and 4; doxorubicin 50 mg/m2 IV continuous infusion over 72 hours; and cyclophosphamide 500 mg/m2 IV on day 1; every 21 days if blood counts have recovered) for a median of four cycles while pregnant. Chemotherapy was held after 35 weeks to avoid maternal neutropenia at the time of delivery. All women had live births, one child had Down syndrome, and two children had congenital anomalies. One woman died of pulmonary embolism after a cesarean delivery. At MDACC, pregnant women with breast cancer continue to be treated with FAC chemotherapy during their second and third trimesters of pregnancy.
The European registry published a recent report detailing outcomes of 413 women who received systemic chemotherapy during pregnancy. Multiple regimens were used, including taxanes, Cyclophosphamide, Methotrexate, Fluorouracil (CMF), and anthracyclines. There was no difference in obstetric complications between women receiving chemotherapy during pregnancy and those who did not. Despite a similar rate of premature deliveries between the two groups, infants exposed to chemotherapy in utero had a lower birth weight as well as an increased risk of complications compared to infants with no exposure to chemotherapy in utero. The authors concluded that a full-term delivery seemed to be paramount to decrease the risk of infant complications (12).
A systematic review of the use of taxanes in pregnancy conducted by Zagouri et al looked at 50 women with breast cancer who received various taxane regimens after the first trimester. They reported that 76% of infants had a normal Apgar score, and at the 16-month follow-up, 90% of the children were completely healthy. One child had recurrent otitis media, one child had immunoglobulin (Ig) A deficiency and constipation, and one child had delayed speech (13). Given the scarcity of the evidence and the lack of long-term follow-up for the children, the routine use of taxanes in pregnant patients with breast cancer cannot be recommended. For our node-positive patients, taxanes are given after delivery.
The routine use of tamoxifen in pregnant patients with breast cancer is not recommended, given animal data suggesting that it could be teratogenic, as well as case reports in humans describing congenital malformations. These include Goldenhar syndrome, ambiguous genitalia, vaginal bleeding, and spontaneous abortions (2).
Trastuzumab use in pregnancy is currently not recommended given multiple reports of oligohydramnios and anhydramnios in the literature (2). Lapatinib exposure during pregnancy was described in one patient who received lapatinib until 11 weeks of gestation, then discontinued the drug. The pregnancy was uneventful, and there were no reported maternal or fetal outcomes (2). There are no current reports on the use of pertuzumab or ado-trastuzumab-emtansine during pregnancy.
There are mixed results in describing prognosis of PABC across the literature. In a series of 121 cases of breast cancer diagnosed during pregnancy, Ribeiro et al reported a 5-year survival rate of 39%. A case-control study from Saudi Arabia compared 28 patients with PABC to 84 women without PABCs and showed no difference in relapse-free or overall survival between the two groups. Beadle et al also showed no difference in locoregional recurrence, distant metastasis, and overall survival between the 104 women with PABC and their 564 non–pregnancy-associated counterparts treated at MDACC between 1973 and 2006 (2). Litton et al reported data on 75 patients treated for PABC on a protocol at MDACC with FAC chemotherapy during the second and third trimesters of pregnancy. In this controlled setting, with all patients receiving similar evaluation and treatment, PABC was associated with improved disease-free and overall survival compared to controls (14). These results show that by using a multidisciplinary approach to the management of PABC, we are able to provide patients with at least as favorable outcomes as their nonpregnant counterparts.
The pregnant patient with breast cancer patient should be referred to a high-risk obstetrician skilled in maternal-fetal medicine, who will be charged with monitoring the health of the mother and the fetus while undergoing cancer therapy.
Prior to initiating treatment, ultrasound is used to determine fetal viability and gestational age and expected date of delivery because both will have a significant effect on treatment planning. In our practice, ultrasound is performed before every cycle of chemotherapy to assess fetal growth and development. Amniocentesis may be recommended by the maternal/fetal health team if the fetus is thought to be at higher-than-average risk for karyotype abnormalities or if there are abnormalities detected by ultrasound that should be investigated further. Although not part of the routine evaluation, amniocentesis may be necessary to assess fetal lung maturity, particularly if early induction of labor is being considered.
Timing of delivery should be optimized with relation to the systemic treatment of the breast cancer, occurring no less than 2 weeks after the last dose of anthracycline-based chemotherapy, to minimize the effects of cytopenias (11). Planned inductions of labor and cesarean deliveries are often done to minimize the risk of pregnancy-associated complications (10).
Given that many chemotherapeutic agents are excreted in breast milk, they can carry a risk of complications to the infant. Therefore, breast feeding should be avoided during the administration of chemotherapy, biologic agents, endocrine therapy, and radiation therapy.
Of 57 children born to mothers who underwent chemotherapy for breast cancer in the second or third trimesters, MDACC has reported that the majority of children were healthy and had no developmental delays, with the exception of one child born with Down syndrome (11). A survey was sent to parents and guardians of children who were exposed to chemotherapy in utero to assess the child’s health, development, and performance in school. Children’s age ranged between 2 and 157 months, and only 2 of 40 evaluated by survey required special attention in school. One child had Down syndrome; the other had attention-deficit disorder. However, longer follow-up of these children will be needed to evaluate possible late side effects, such as impaired cardiac function and fertility. Another study by Aviles et al described similar outcomes for a cohort of 84 children born to mothers who received chemotherapy for hematologic malignancies while pregnant (15). They also evaluated 81 of these children for cardiac toxicity, using clinical evaluation and echocardiography, every 5 years after birth until 29 years of age. There was no evidence of cardiac dysfunction among the children, ranging in age from 9.3 to 29.5 years (mean 17.1 years) (15).
A number of case series do not appear to support the previously held belief that pregnancy termination improves the survival of pregnant patients with breast cancer. In contrast, there appears to be a trend toward shorter survival with termination of pregnancy (2). A pregnant woman with breast cancer must be fully aware of the evidence, or lack thereof, regarding pregnancy termination and survival. In situations of known or suspected fetal teratogenesis or if maternal health is in jeopardy, pregnancy termination may be an appropriate medical recommendation.
PREGNANCY AFTER A DIAGNOSIS OF BREAST CANCER
Of the women diagnosed with breast cancer between 2007 and 2011 in the Surveillance Epidemiology and End Results (SEER) database, 1.8% were 20 to 34 years of age, and 9.3% were 35 to 44 years of age.
According to the National Center for Health Statistics, the average age of first-time mothers increased from 21.4 years in 1970 to 25 years in 2006 and to 25.8 years in 2012. From 1970 to 2006, the proportion of first births to women aged 35 years and older increased by almost eight times. In 2006, about 1 of 12 first births was to women aged 35 years and older compared with 1 of 100 in 1970. In the 2012 update, the birth rate for women in their early 20s declined to a record low, but continued to increase in women aged 30 to 44 years old.
Therefore, younger women diagnosed with breast cancer may not have had children at the time of their breast cancer diagnosis and may seek to do so after their breast cancer treatment is completed. Of course, patients with breast cancer who have had children before their diagnosis also may wish to have additional children after treatment.
Chemotherapy-related amenorrhea (CRA) is variably defined as cessation of menstruation for 3 to 12 months in women who have been exposed to chemotherapy (16). The incidence of CRA varies with age, cytotoxic agent used, and cumulative cytotoxic dose (16). A study by Goodwin et al looked at 183 women who underwent systemic therapy, including chemotherapy and tamoxifen for breast cancer. Multivariate analysis showed that age, chemotherapy, and tamoxifen were all predictors of menopause in patients with breast cancer receiving systemic therapy (17). Taxanes may result in a higher rate of CRA in the first year but have not been shown to cause a longer duration of CRA; this effect is primarily seen in older women, and when age is controlled for, adding a taxane appears to have little-to-no effect on subsequent risk of CRA (18). Lee et al reviewed the risk of permanent amenorrhea by chemotherapy regimen and patient age (19).
The current method recommended by the American Society of Clinical Oncology for fertility preservation is controlled ovarian stimulation followed by oocyte or embryo cryopreservation (20,21). The efficacy of ovarian suppression by a gonadotropin-releasing hormone analogue (GNRHa) at reducing the risk of CRA has been evaluated in multiple studies. Gerber et al randomized 60 patients with hormone receptor–negative breast cancer to chemotherapy with or without goserelin. There was no difference in amenorrhea at 6 months between the two groups, and all but one patient had regular menses at 2 years (22). The PROMISE-GIM6 (Prevention of Menopause Induced by Chemotherapy: A Study in Early Breast Cancer Patients-Gruppo Italiano Mammella 6) trial randomized 281 patients with stage I to III breast cancer to chemotherapy with or without triptorelin. There was a significant decrease in the incidence of early menopause in the triptorelin group (26% vs 9%) (23). The POEMS/SWOG (Southwest Oncology Group) 0230 trial enrolled 257 hormone receptor–negative premenopausal patients with stage I to IIIA breast cancer randomized to receive cyclophosphamide-containing chemotherapy with or without goserelin. There was a decrease in the risk of premature ovarian failure (POF) at 2 years in the goserelin group (22% vs 8%), and more successful pregnancies were noted in the goserelin arm. A meta-analysis by Del Mastro et al revealed a significant decrease in the risk of POF, with an odds ratio of 0.43, in patients receiving GNRHa (24).
A number of reviews have concluded that pregnancy after a diagnosis of breast cancer does not worsen survival (25,26,27). In a retrospective case-control study by Mueller et al, data from three SEER populations were linked to vital records data to identify women under the age of 45 at diagnosis who had live birth 10 months or longer after diagnosis. When these women were matched to nonpregnant women with a history of breast cancer, women who became pregnant after a diagnosis of breast cancer had a decreased risk of dying as compared with women who did not become pregnant (28). The improved survival noted in this and other studies may reflect a “healthy-mother effect,” whereby women who become pregnant after a diagnosis of breast cancer may have already been at decreased risk of recurrence.
Women who are considering pregnancy after a diagnosis of breast cancer should understand that most data come from retrospective case-control studies in different populations and with different data collection techniques. Women with a history of breast cancer must be aware of their personal risk of recurrence and should weigh this against their desire to have a child. Although it has been suggested that women should wait 2 years after their breast cancer diagnosis before becoming pregnant, there are no data suggesting that a pregnancy in the first 2 years increases the risk of recurrence; rather, data indicate that this is the period of increased recurrence regardless of pregnancy (29).
MALE BREAST CANCER
Male breast cancer is rare, accounting for less than 1% of male cancers. It was estimated that 2,360 new cases of breast cancer would be diagnosed in men in the United States in 2014 and that 430 men would die of the disease (30). A large population-based study done by Giordano et al suggested that the incidence of male breast carcinoma is increasing, from 0.86 to 1.08 per 100,000 population between 1973 and 1988, albeit at a slower rate than that of women (31). Men with BRCA mutations are at higher risk of male breast cancer compared to the general population, with a lifetime risk of 4% to 40% for BRCA 2 mutation carriers and up to 4% for BRCA 1 mutation carriers (32). Other risk factors for development of male breast cancer include testicular abnormalities, infertility, Klinefelter syndrome, positive family history, benign breast conditions, radiation exposure, increasing age, and Jewish ancestry. Other studies have also identified PTEN mutations, CHEK 2 mutations, obesity, and gynecomastia as potential risk factors for male breast cancer. There is a 2.5-fold increase in risk in men who have female relatives with breast cancer (32,33).
The most common presenting symptom for men with breast cancer is a painless lump. Other symptoms include nipple retraction, local pain, nipple ulceration, nipple bleeding, and nipple discharge (33). The mean age of diagnosis is 67 years, compared to 62 years for women with breast cancer (32). Male breast cancer is more likely to have a delay in diagnosis when compared to women; this is thought to in part account for the later stage of disease and greater tumor size with lymph node involvement at diagnosis (33).
A biopsy should be performed of any suspicious mass. If breast cancer is diagnosed, the male patient with breast cancer should undergo staging evaluations appropriate for the given tumor stage, based on the AJCC TNM staging system.