PART A: SMALL BOWEL CANCER
Small bowel cancer is a rare malignancy representing approximately 3% of gastrointestinal neoplasms (1). In 2014, it was estimated that 9,160 new cases of small bowel cancer and 1,210 small bowel cancer–related deaths would occur (1). The two most common histologies seen in cancers of the small intestine are carcinoids and adenocarcinomas (2). Because of the nonspecific clinical presentation of small bowel adenocarcinoma and the difficulty in imaging the small bowel, most patients with small bowel adenocarcinoma present with lymph node involvement or distant metastases. Even in patients with localized disease who undergo resection with curative intent, the prognosis is poor, and no studies have yet demonstrated a clear benefit from adjuvant therapy. However, there have been some recent advances in the use of chemotherapy as palliative treatment. In this chapter, the epidemiology, diagnosis, and treatment of small bowel cancers, in particular small bowel adenocarcinoma, are reviewed.
Based on an analysis of the Surveillance, Epidemiology, and End Results (SEER) database, the age-adjusted incidence rate for small bowel cancers has slowly increased from 0.9 per 100,000 persons in the years 1973 to 1982 to 1.8 per 100,000 persons in the years 2000 to 2004 (3,4). The majority of this increase has been attributed to an increase in the incidence of carcinoid tumors. A recent analyses of 67,643 patients with small bowel malignancies between 1973 and 2004 using the National Cancer Data Base and SEER showed carcinoids to be dominant with 37.4% cases compared to 36.9% cases of adenocarcinomas (5). The incidence of histologic subtypes varies in the different sections of the small intestine, with adenocarcinomas representing 80% of duodenal cancers and carcinoids representing 60% of ileal cancers.
The incidence of small bowel adenocarcinoma peaks in the seventh and eighth decades of life, with a mean age at diagnosis of 65 years. A slightly increased incidence is seen in men and blacks (6).
One of the more interesting aspects of small bowel adenocarcinoma is its rarity in comparison to large intestine adenocarcinoma. Even though the small intestine represents approximately 70% to 80% of the length and over 90% of the surface area of the alimentary tract, the incidence of small bowel adenocarcinoma is 30-fold less than the incidence of colon adenocarcinoma. Numerous theories have been proposed to explain the small intestine’s relative protection from the development of carcinoma. Proposed protective factors have centered on two concepts. First, the rapid turnover time of small intestinal cells results in epithelial cell shedding prior to the necessary acquisition of multiple genetic defects. Second, the small bowel’s exposure to the carcinogenic components of our diet are limited due to rapid small bowel transit time, the lack of bacterial degradation activity that occurs in the small bowel, and the relatively dilute, alkaline environment of the small bowel. In a population-based comparison of adenocarcinomas of the large (n = 261,521) and small (n = 4,518) intestine identified from the SEER registry, small bowel adenocarcinomas demonstrated a distinctly worse stage-adjusted cancer-specific survival compared to colorectal adenocarcinomas (7).
The small intestine is divided into three sections. The duodenum represents the first 25 cm of the small intestine and is subdivided into four anatomic segments. The proximal portion of the first (ascending) segment of the duodenum is intraperitoneal, and then the distal portion, as well as the rest of the duodenum, becomes retroperitoneal. The second (descending) segment of the duodenum contains the ampulla of Vater, through which the pancreatic and biliary secretions exit. The third (horizontal) segment of the duodenum is the longest, and as it crosses the left border of the aorta, the fourth (ascending) segment of the duodenum begins. The duodenal-jejunal junction is characterized by the attachment of the suspensory ligament of Treitz. The next segment of the small bowel, the jejunum, is approximately 2.5 m long, and the final segment, the ileum, is approximately 3.5 m long.
Little is known about the etiology of small bowel adenocarcinoma. As seen in colorectal adenocarcinomas, adenocarcinomas of the small intestine undergo a similar phenotypic adenoma-carcinoma transformation (8,9,10). An increase in the size of small bowel adenomas and the presence of a villous histology are risk factors for the development of invasive adenocarcinoma.
Common underlying genetic or environmental factors of both large and small intestine adenocarcinomas have been suggested by studies that have demonstrated an increased risk of small bowel adenocarcinoma in patients with colon adenocarcinoma and vice versa (11). In small bowel adenocarcinoma, microsatellite instability occurs at a similar rate to that seen in colorectal cancer (CRC). In a study of 89 patients with small bowel adenocarcinoma identified from a Swedish population-based cancer registry, the rate of microsatellite instability was 18% (12). This result along with the known clinical association between hereditary nonpolyposis colorectal cancer (HNPCC) syndrome and small bowel adenocarcinoma indicate that in a subset of patients with small bowel adenocarcinoma, a germline mutation in one of the mismatch repair proteins contributes to carcinogenesis.
The possible role of pancreaticobiliary secretions in the development of adenocarcinoma of the duodenum has been suggested by the anatomic clustering of duodenal carcinomas in the periampullary area. For example, in patients with familial adenomatous polyposis (FAP), 80% of small intestinal adenocarcinomas will occur in the second portion of the duodenum. One study evaluating 213 cases of duodenal carcinomas identified from the Los Angeles County tumor registry determined that 57% of the cases originated in the second part of the duodenum (13).
A number of case-control studies have analyzed associations between environmental and dietary factors and the development of small bowel adenocarcinoma. Two studies have demonstrated that there is an association between the ingestion of smoked or salt-cured foods and the development of small bowel adenocarcinoma (14,15). An association between tobacco use and cancer risk has been inconsistently demonstrated. Case-control studies have demonstrated an association between an increased risk of small bowel adenocarcinoma and high alcohol intake, high sugar intake, high red meat intake, low fiber intake, celiac disease, peptic ulcer disease, and prior cholecystectomy (14,15,16,17,18). Studies of the relationship with obesity have been conflicting, although a recent case-cohort study of 500,000 subjects with 134 incident cases of small bowel cancer showed a statistically nonsignificant trend toward increased risk in subjects with high body mass index (BMI) (hazard ratio [HR] 1.5 [95% CI, 0.76-2.96] for BMI >27.5 kg/m2 compared with 22.6-25.0 kg/m2) (19).
The genetic cancer syndromes HNPCC, FAP, and Peutz-Jeghers syndrome (PJS) are all associated with small bowel adenocarcinoma. The estimated lifetime risk for small bowel adenocarcinoma is 1% to 4% in patients with HNPCC, 5% in those with FAP, and 13% in those with PJS (20,21,22,23). Patients with HNPCC develop small bowel adenocarcinoma at a younger age, with a median age at diagnosis of 49 years. Patients with PJS, an autosomal dominant polyposis disorder characterized by multiple hamartomatous polyps throughout the intestinal tract, have a markedly increased risk for small bowel adenocarcinoma, with one meta-analysis demonstrating a 520-fold increased relative risk (24). Duodenal adenomas are seen in approximately 80% of patients with FAP, and regular endoscopic screening for the development of adenocarcinoma is required for these patients. The optimal frequency of endoscopic screening depends on a number of factors, such as the number of polyps, polyp size, polyp histology, and amount of dysplasia present (20). With the early use of colectomy in patients with FAP, duodenal adenocarcinomas and desmoid tumors are now a more common cause of death in this population than cancer arising from the colorectum.
Inflammatory bowel disease, particularly Crohn disease, is associated with the development of small bowel adenocarcinoma. The increase in risk varies depending on both the extent and duration of small bowel involvement. In one study, the cumulative risk of small bowel adenocarcinoma in patients with Crohn disease was 0.2% at 10 years and 2.2% at 25 years (25). Because Crohn disease frequently involves the ileum, 70% of the small bowel cancers in patients with Crohn disease will occur in the ileum. Patients with Crohn disease who develop small bowel adenocarcinoma appear to have a worse prognosis, with one study of 37 patients with small bowel adenocarcinoma demonstrating significantly shorter overall survival (OS) in the patients with Crohn disease (26).
Early limited data has begun to accumulate regarding molecular characterization of small bowel adenocarcinomas. Accumulation of genetic defects such as loss of e-cadherin and smad4 and mutations in KRAS, P53, and SMAD4 have been implicated in the adenoma-dysplasia-carcinoma sequence of small bowel adenocarcinomas (2). In a pivotal study comparing chromosomal copy number aberrations in 85 gastric, colorectal, and small bowel adenocarcinomas, hierarchical clustering revealed a substantial overlap of sba copy number profiles with matched colorectal adenocarcinomas but less overlap with profiles of gastric adenocarcinomas, indicating a genetic profile similar to CRC (27). Large screening of somatic mutations in 83 patients revealed KRAS, TP53, APC, SMAD4, PIK3CA, erbb2, braf, and fbxw7 mutations in >5% of small bowel adenocarcinomas (24). Understanding the biology of small bowel adenocarcinomas may lead to the possibility of developing targeted therapy in this rare cancer.
The symptoms associated with small bowel adenocarcinoma are nonspecific and frequently do not occur until advanced disease is present. The most commonly reported symptoms are abdominal pain (45%-76% of patients), nausea and vomiting (31%-52%), weight loss (22%-29%), and gastrointestinal bleeding (8%-34%). Delays in diagnosis are common, with one retrospective study reporting a mean delay of 7.8 months from the time of initial physician evaluation until a final diagnosis was made (28). According to the National Cancer Database, 39% of patients present with stage I/II disease, 26% present with stage III disease, and 32% present with stage IV disease (29).
Given the nonspecific presenting symptoms, a high index of suspicion is a crucial first step in diagnosis. Because imaging of the small intestine is difficult, multiple tests may be needed. However, with the availability of wireless capsule endoscopy, the need for older small bowel imaging techniques has declined.
A barium small bowel follow-through study has been the radiographic gold standard for small bowel evaluation. In patients with advanced-stage disease, this technique has a sensitivity of approximately 60% for diagnosing small bowel tumors. Enteroclysis, in which contrast material is infused directly into the small intestine through a nasogastric tube, provides a slightly higher sensitivity than small bowel follow-through. Endoscopic evaluation of the small intestine, or enteroscopy, requires expertise and is frequently unable to evaluate the entire small intestine.
The incorporation of wireless capsule endoscopy, which was approved by the US Food and Drug Administration in 2001, has allowed a much simpler and improved method for evaluating the lumen of the small intestine. This technique has primarily been applied to the evaluation of obscure gastrointestinal bleeding, for which it has shown superiority over other imaging and endoscopy techniques (30). In one study evaluating capsule endoscopy in 60 patients with suspected small bowel pathology but without gastrointestinal bleeding, the overall diagnostic yield of capsule endoscopy was 62% (31). In that study, all patients had undergone upper and lower gastrointestinal endoscopy, and many had undergone enteroclysis, small bowel follow-through, push enteroscopy, and abdominal computed tomography (CT). In a large single-center retrospective review of 562 patients who underwent capsule endoscopy, small bowel tumors were found in 8.9% of cases (32). The major limitations of capsule endoscopy are that no tissue sampling can be conducted and that the patients cannot have bowel obstruction, which could result in the capsule’s becoming trapped in the bowel.
Three-dimensional imaging with either CT or magnetic resonance imaging (MRI) is useful in identifying locoregional lymph node involvement and the presence of distant metastatic disease. For tumors of the duodenum, endoscopic ultrasonography can be useful in assessing both the tumor and nodal status. Although not directly studied for duodenal adenocarcinomas, endoscopic ultrasonography has been demonstrated to improve staging accuracy for both ampullary and pancreatic cancers.
The TNM (tumor, node, metastasis) staging system for small bowel adenocarcinoma is shown in Table 23-1 (33). In a study of 4,995 patients who were diagnosed with small bowel adenocarcinoma between 1985 and 1995 (identified in the National Cancer Database), the 5-year disease-specific survival (DSS) rate was 65% for patients with stage I disease, 48% for patients with stage II disease, 35% for patients with stage III disease, and 4% for patients with stage IV disease (29). A multivariate analysis from this study identified age >75 years, primary duodenal tumor site, non–cancer-directed surgery, and higher-stage disease as poor prognostic factors. Though significant on a univariate analysis, a poorly differentiated histology was not a significant prognostic factor on multivariate analysis (P = .089). In other studies, the histopathologic factors reported to be correlated with poor survival were poorly differentiated histology, positive margins, lymphovascular invasion, lymph node involvement, and T4 tumor stage (26,34,35,36). The 5-year OS rates from various single-institution studies for resected small bowel adenocarcinoma are presented in Table 23-2.
| Primary Tumor (T) | |||
| TX | Primary tumor cannot be assessed | ||
| T0 | No evidence of primary tumor | ||
| Tis | Carcinoma in situ | ||
| T1a | Tumor invades lamina propria | ||
| T1b | Tumor invades submucosa | ||
| T2 | Tumor invades muscularis propria | ||
| T3 | Tumor invades through the muscularis propria into the subserosa or into the nonperitonealized perimuscular tissue (mesentery or retroperitoneum) with extension 2 cm or lessa | ||
| T4 | Tumor perforates the visceral peritoneum or directly invades other organs or structures (includes other loops of small intestine, mesentery, or retroperitoneum >2 cm and abdominal wall by way of serosa; for duodenum only, invasion of pancreas or bile bile duct) | ||
| Regional Lymph Nodes (N) | |||
| NX | Regional lymph nodes cannot be assessed | ||
| N0 | No regional lymph node metastasis | ||
| N1 | Metastasis in 1-3 regional lymph nodes | ||
| N2 | Metastasis in 4 or more regional lymph nodes | ||
| Distant Metastasis (M) | |||
| M0 | No distant metastasis | ||
| M1 | Distant metastasis | ||
| Stage Grouping | |||
| Stage 0 | Tis | N0 | M0 |
| Stage I | T1 | N0 | M0 |
| T2 | N0 | M0 | |
| Stage IIA | T3 | N0 | M0 |
| Stage IIB | T4 | N0 | M0 |
| Stage IIIA | Any T | N1 | M0 |
| Stage IIIB | Any T | N2 | M0 |
| Stage IV | Any T | Any N | M1 |
| First Author | Period | Tumor Location | Number of Patients | Overall Survival | |
|---|---|---|---|---|---|
| Median (Months) | 5 Year (%) | ||||
| Agarawal | 1971-2005 | Small intestine | 30 | 56 | 45 |
| Kelsey | 1975-2005 | Duodenum | 25 | NR | 64 |
| Wu | 1983-2003 | Small intestine | 45 | NR | 27 |
| Swartz | 1994-2003 | Duodenum | 14 | 41 | 44 |
| Dabaja | 1978-1998 | Small intestine | 142 | 36 | 29 |
| Czaykowski | 1990-2000 | Small intestine | 19 | 39 | NR |
| Talamonti | 1977-2000 | Small intestine | 26 | 40 | 42 |
| Abrahams | 1978-1999 | Small intestine | 37 | NR | 52 |
| Brucher | 1985-1998 | Small intestine | 22 | NR | 45 |
| Bakaeen | 1976-1996 | Duodenum | 68 | NR | 54 |
| Rose | 1983-1994 | Duodenum | 42 | NR | 60 |
| Cunningham | 1970-1991 | Small intestine | 19 | 23 | 32 |
| Frost | 1960-1989 | Small intestine | 22 | NR | 32 |
For patients with localized disease, complete removal of the tumor with negative surgical margins and local lymph node removal are critical for a potentially curative resection. For jejunal and ileal lesions, an oncologically successful resection requires a wide local excision with lymphadenectomy. Lesions located in the duodenum generally require a pancreaticoduodenectomy; however, for small distal lesions in the third and fourth portions of the duodenum, a wide local excision may be an option. In a surgical series of 68 patients with duodenal adenocarcinoma, no differences in the 5-year OS rates, local recurrence rates, or margin-negative resection rates were seen between the 50 patients who underwent pancreatic resections and the 18 patients who underwent distal duodenal segmental resections (37). The presence of locoregional lymph node involvement should not deter surgical intervention because well over one-third of patients will survive long term (29,38). This is in contrast to patients with lymph node–positive pancreatic cancer, of whom only 7% survive 5 years (39). As is seen with colon cancer, the total lymph nodes (TLNs) assessed during surgery and the number of positive lymph nodes (PLNs) have prognostic implications in small bowel adenocarcinomas. In a SEER registry retrospective review of 1,991 patients, the 5-year DSS for patients with stage II disease appeared to be associated with the TLNs assessed (44%, 69%, and 83% for 0 TLNs, 1 to 7 TLNs, and >7 TLNs, respectively) (40). Furthermore, the 5-year DSS with stage III disease was associated with the number of PLNs (58% and 37% for <3 PLNs and ≥3 PLNs, respectively) (40).
Recurrence after potentially curative resection of small bowel adenocarcinoma occurs most commonly at distant sites. In a series of 146 patients who underwent resection for small bowel adenocarcinoma, 56 patients relapsed at a median of 25 months, with the sites of relapse reported as distant in 59%, peritoneum in 20%, abdominal wall in 7%, and local in 18% (41). In a second study of 30 patients who underwent potentially curative resection for small bowel adenocarcinoma, 21 patients experienced a relapse, with the sites of relapse being the liver in 67% of the patients, lung in 38%, retroperitoneum in 29%, and peritoneum in 25% (34).
Patients with duodenal adenocarcinoma have a higher rate of locoregional failure than for jejunal and ileal adenocarcinoma, with one study reporting a 39% rate of locoregional failure among 31 patients after curative resection of duodenal adenocarcinoma (42). In that study, positive margin status was the strongest predictor of local recurrence, with 4 of the 5 patients who had a margin positive resection developing a local recurrence. However, distant recurrences are still predominant, with a retrospective review of recurrence patterns in 67 patients with resected duodenal adenocarcinoma revealing local recurrences in 33% of the patients and distant recurrences in 67% (43).
Currently, there is no evidence demonstrating a benefit from adjuvant therapy in patients with small bowel adenocarcinoma who undergo potentially curative resection. However, owing to the rarity of small bowel adenocarcinoma, only a limited number of primarily small retrospective studies have been conducted (Table 23-3). Selection bias is the major limitation of these retrospective studies because the patients selected to receive adjuvant therapy were the patients believed to be at highest risk for disease recurrence. One prospective phase III study conducted by the European Organization for Research and Treatment of Cancer randomized 93 eligible patients with periampullary carcinoma (defined as adenocarcinoma of the distal common bile duct, ampulla of Vater, or duodenum) to receive either no adjuvant therapy or concurrent 5-fluorouracil (5-FU) and radiation therapy (44). The 5-year OS rates were similar in the two groups, but 30% of the patients assigned to receive adjuvant therapy did not actually receive it, and no description of the results in the duodenal adenocarcinoma subgroup were reported.
| First Author | Time Period | Study Type | Tumor Location | Type of Adjuvant Therapy | Number of Patients | Median Overall Survival (Months) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | No Adjuvant Therapy | Adjuvant Therapy | No Adjuvant Therapy | Adjuvant Therapy | P Value | |||||
| Agrawal | 1971-2005 | Retrospective review | Small bowel | Not specified | 30 | 19 | 11 | 41 | 56 | NR |
| Kelsey | 1975-2005 | Retrospective review | Duodenum | 5-FU/radiation | 32 | 16 | 16 | 44%a | 57%a | 0.42 |
| Fishman | 1986-2004 review | Retrospective | Small bowel | Not specified | 60 | 45 | 15 | 28 | 22 | NR |
| Dabaja | 1978-1998 | Retrospective review | Small bowel | Not specified | 120 | 62 | 58 | 36 | 19 | 0.49 |
| Klinkenbiji | 1987-1995 | Randomized phase III trial | Periampullary | 5-FU/radiation | 93 | 49 | 44 | 40 | 40 | 0.74 |
| Sohn | 1984-1996 | Retrospective review | Duodenum | 5-FU/radiation | 48 | 37 | 11 | 35 | 27 | 0.73 |
| Overman | 1990-2008 | Retrospective review | Small Bowel | Chemo/radiation | 54 | 24 | 30 | NR | NR | 0.84 |
| Halfdanarson | 1970-2005 | Retrospective review | Small Bowel | Chemo/radiation | 58 | 60 | 45 | 26.5 | 30.2 | 0.36 |
In a series by Kelsey et al, no differences in the 5-year OS rates were seen between the patients who did or did not receive adjuvant therapy after resection of duodenal adenocarcinoma. However, in the subgroup of patients who had undergone a margin-negative resection, the 5-year OS rate was 53% in the patients who underwent resection only and 83% in the patients who had resection and adjuvant chemoradiation therapy (P = .07) (42). A trend toward improvement of disease-free survival and OS was seen in patients receiving adjuvant therapy in a retrospective series at MD Anderson Cancer Center (n = 54) (45). However, in the subgroup analyses in patients with high-risk disease, defined by a lymph node ratio ≥10%, adjuvant therapy was associated with significant improvement in OS (45). In contrast, in a single-institution retrospective review at Mayo Clinic, no benefit of adjuvant chemotherapy or chemoradiotherapy was seen in patients with resected small bowel adenocarcinoma (46).
Limited data are available regarding a neoadjuvant (preoperative) treatment approach for duodenal adenocarcinoma. In one report in which 11 patients underwent neoadjuvant chemoradiation therapy followed by resection for duodenal adenocarcinoma, a complete pathologic response was seen in 2 patients, and none of the 11 patients had histopathologic nodal involvement at the time of surgery (42).
At the University of Texas MD Anderson Cancer Center, patients with high-risk, resected small bowel adenocarcinoma are typically offered postoperative adjuvant chemotherapy. In general, patients who are considered to be at high risk are those with lymph node involvement and positive resection margins. The lack of proven benefit from adjuvant chemotherapy for this tumor type must be discussed with the patient. However, the rationale for considering adjuvant chemotherapy is based on
The known poor prognosis of patients with high-risk disease
The predominantly systemic relapse pattern for small intestinal adenocarcinoma
The proven activity of chemotherapy in the treatment of metastatic small intestinal adenocarcinoma
The known benefit of adjuvant chemotherapy in large intestinal adenocarcinoma, which appears to have a number of similarities to small intestinal adenocarcinoma
The extremely limited amount of high-quality data to support or refute the role of adjuvant therapy for small bowel adenocarcinoma
Based on the substantial activity of a 5-FU and platinum combination in the metastatic disease setting, we generally utilize the combination of capecitabine and oxaliplatin (CAPOX) as adjuvant therapy for nonmetastatic small bowel adenocarcinoma. In addition to systemic chemotherapy, radiation therapy is considered for patients with duodenal adenocarcinoma who are at high risk for a local recurrence based on the presence of positive margins or T4 disease.
In general, chemotherapy for metastatic small bowel adenocarcinoma has been based on the principles used for treating colon cancer. Several single-institution retrospective series have demonstrated a survival benefit in patients with metastatic or unresectable small bowel adenocarcinoma who received chemotherapy when compared to patients who did not receive chemotherapy (41,47).
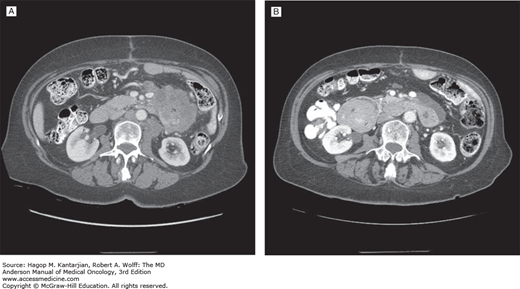
Most of the studies evaluating chemotherapy for small bowel adenocarcinoma have been retrospective, with only four prospective phase II studies reported (Table 23-4). One multicenter study conducted by the Eastern Cooperative Oncology Group reported on the combination of 5-FU, doxorubicin, and mitomycin C (FAM) in 39 patients with adenocarcinomas of the duodenum, jejunum, ileum, or ampulla of Vater. The overall response rate was 18%, with a median OS time of 8 months (48). A single-institution study conducted at MD Anderson evaluated CAPOX in 30 patients with metastatic or locally advanced small bowel or ampullary adenocarcinomas. The overall response rate was 50%, with a median time to progression of 9.8 months and an OS time of 20.3 months (49). An example of a response to CAPOX chemotherapy in a patient treated in that study is shown in Fig. 23-1. In 33 patients treated with continuous infusional 5-FU and leucovorin in combination oxaliplatin (modified 5-FU and oxaliplatin [FOLFOX] regimen), the objective response rate was 48.5%, and the median OS was 15.2 months (50). Another prospective, multicenter, first-line study (n = 23) using CAPOXIRI (capecitabine, oxaliplatin, and irinotecan) showed a response rate of 39% and a median survival of 12.7 months (51).
| First Author | Year | Study Type | Disease Status | Number of Patients | Type of Chemotherapy | Overall Response Rate (%) |
|---|