Skin and Soft-Tissue Syndromes
Skin infections should be distinguished from exanthems, which are rashes associated with a generalized febrile disease and are discussed in Chapter 11. A primary skin infection is one in which the principal and original manifestation of the infection is in the skin. Examples of such infections that are discussed below include cellulitis, gangrene, impetigo, pustules, boils, abscesses, scalded skin syndrome, tinea, and skin ulcers. A secondary skin infection is one occurring because of a change in the skin’s protective mechanism. Examples include acne, burns, diaper dermatitis, and wounds, either surgical or traumatic. Some skin infections only involve the epidermis or dermis, whereas others may also involve the underlying subcutaneous fat and fascia, in which case the broader term, soft-tissue infection, is used. Infection of muscle (pyomyositis) is addressed in Chapter 16. Animal and human bites are discussed in Chapter 21.
Normal Skin Flora
Normally, bacteria of the skin can be found on the surface, in the hair follicles, or beneath the superficial cells of the stratum corneum.1 Skin flora can be divided into two groups: resident and transient. The resident flora exist in relatively stable numbers and consist primarily of Staphylococcus epidermidis, micrococci, and aerobic and anaerobic diphtheroids, such as Propionibacterium species.2 Transient flora are introduced from the environment and usually only reside on the skin temporarily. The most important of the transient flora are Staphylococcus aureus and group A β-hemolytic streptococcus. S. aureus is found so frequently (approx. 30%) in the nose, axilla, groin, perineum, and the newborn umbilicus that it is sometimes regarded as normal flora of these areas.3,4,5 Children with underlying skin disease such as atopic dermatitis have much higher rates of colonization with S. aureus (approx. 90%).6 Hospitalized persons, especially those in intensive care units, often become colonized with a wide variety of organisms.7 Exposure to antibiotics, even as an outpatient, changes the composition of skin flora, primarily increasing colonization with Candida.8
Most superficial skin infections occurring in outpatients are caused by either S. aureus or group A streptococci, although occasionally other organisms are implicated.2 Among hospitalized patients, a greater array of organisms is seen. Among 1562 bacterial isolates recovered from hospitalized patients with skin and soft tissue infections in the United States and Canada, the most common pathogens were S. aureus (43%), Pseudomonas aeruginosa (11%), Enterococcus spp. (8%), Escherichia coli (7%), Enterobacter spp. (5%), and β-hemolytic streptococci (5%).9
Cellulitis
Cellulitis is defined as a localized inflammation of the skin, recognized by an area of redness and warmth. Fever may be present, and underlying subcutaneous tissue is often involved. Note that this broad definition includes noninfectious diseases that may mimic bacterial cellulitis. Lymphangitis, which may accompany cellulitis, is a thin line of redness typically extending from an infected wound along the route of the lymphatic drainage. If the erythema and warmth are more generalized, the diagnosis of scarlet fever or scalded skin syndrome should be considered.
Cellulitis is a diagnosis that can often be subclassified according to its location, which gives a clue to the etiology, as described later. Cellulitis can also be described by adjectives that imply more severe disease than “simple” cellulitis, which is manifested only by redness, warmth, and swelling of the soft tissues.
Necrotizing Cellulitis
In the past, a distinction was made between necrotizing cellulitis and necrotizing fasciitis.10 In necrotizing cellulitis the skin is involved early with hemorrhage
and necrosis. In necrotizing fasciitis, the skin and subcutaneous tissues are lifted up by dissection of infection along fascial planes, and the skin is pale and shiny. However, the distinction between these two conditions is not always easy, and the precise label is of little importance. Early recognition and urgent operative intervention is critical for both conditions (see section on necrotizing soft-tissue infections).
and necrosis. In necrotizing fasciitis, the skin and subcutaneous tissues are lifted up by dissection of infection along fascial planes, and the skin is pale and shiny. However, the distinction between these two conditions is not always easy, and the precise label is of little importance. Early recognition and urgent operative intervention is critical for both conditions (see section on necrotizing soft-tissue infections).
Crepitant Cellulitis
As discussed in the section on necrotizing soft-tissue infections, crepitance of a cellulitic area should be presumed to be early gangrene.
Location
The location of the cellulitis is very important, and the descriptive diagnosis should always state the location, because there may be a serious infection underneath the cellulitis. The center point of the cellulitis may give a clue to the underlying disease (Table 17-1). Important possible underlying diseases include osteomyelitis, septic arthritis, peritonitis, sinusitis, neck space infections, or deep wound infections, all of which are discussed in other chapters.
TABLE 17-1. POSSIBLE CAUSES OF CELLULITIS IN VARIOUS LOCATIONS | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Cellulitis of an Extremity
Cellulitis of an arm or leg is often associated with local injury and a minor wound. Lymphangitic streaking, local tenderness, erythema, fever, malaise, and tender regional adenopathy are common.2 A poorly defined border of erythema could simply be due to cellulitis deep in the subcutaneous tissue or could signal an underlying osteomyelitis, especially if there is tenderness over the metaphysis (see Chapter 16). Blood cultures are usually negative in simple cellulitis of an extremity, and aspirate cultures are positive in about one-quarter of cases.11,12 Many experts recommend aspirating the leading edge as opposed to the center of the lesion, although a study comparing the two techniques found no difference in the yield.13 Swab cultures of pus give higher yields, but visible pus is not frequently present.
S. aureus is the most common cause, followed by group A streptococcus. In the majority of cases, mild cellulitis of an extremity in a normal child can be treated with an oral antibiotic (e.g., cephalexin)
that covers these two organisms. For immunocompromised children (including neonates) and children with manifestations of a severe infection (such as high fever or toxicity), blood cultures should be obtained, and parenteral therapy (e.g., oxacillin or cefazolin) should be started.
that covers these two organisms. For immunocompromised children (including neonates) and children with manifestations of a severe infection (such as high fever or toxicity), blood cultures should be obtained, and parenteral therapy (e.g., oxacillin or cefazolin) should be started.
Sacral Cellulitis
Infection of a pilonidal cyst is the usual cause of midline sacral cellulitis. It occurs predominantly in males and usually manifests clinically near the end of the second decade of life. Pilonidal cysts are asymptomatic until they become infected. The primary therapy of an infected pilonidal cyst is surgical, either excision or incision and curettage.14 The cyst abscess usually contains mixed anaerobic and aerobic flora, so ampicillin-sulbactam or clindamycin with gentamicin is reasonable perioperative coverage, although healing time is similar whether or not antibiotics are given.15,16
Perianal Cellulitis
This is a relatively common problem in young children, and group A streptococcus is the usual cause. Children usually present with a sharply demarcated area of perianal erythema, pruritis, painful defecation and, occasionally, blood-streaked stools.17 Vaginal involvement may occur in girls. A swab for culture of group A streptococcus should be performed (rapid tests are neither approved nor appropriate for diagnosis of this condition). Treatment is with oral penicillin or amoxicillin.
External Ear
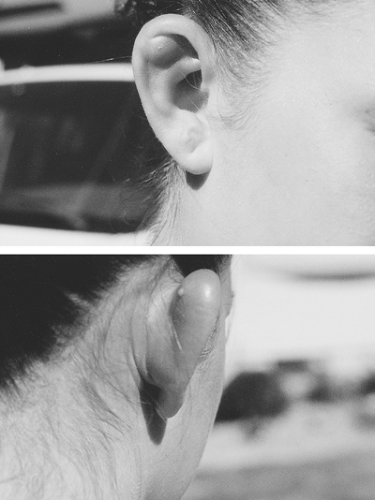
Cellulitis of the ear may be a manifestation of malignant otitis externa (discussed in Chapter 5). More commonly, infection of the pinna is secondary to ear piercing. Infection of the earlobe, which has its own blood supply, is usually a minor problem and responds to removal of the embedded earring and oral antistaphylococcal antibiotics. In contrast, infection of the upper ear cartilage, termed auricular perichondritis, is more difficult to treat (Fig. 17-1).18 P. aeruginosa is the most common cause.19 The ear cartilage does not have its own blood supply, so antibiotics alone are usually ineffective. Early referral to a plastic surgeon for debridement of infected cartilage is appropriate.
Infectious Etiologies
Group A Streptococcus
Group A streptococcus (GAS) and S. aureus are the two most common causes of cellulitis. Streotococcal cellulitis is infrequently associated with fluctuance; the infection may progress rapidly. Perineal streptococcal carriage in surgical personnel has been associated with outbreaks of postoperative wound infections.20 Erysipelas is a form of cellulitis that is almost always due to GAS and involves the more superficial layers of the skin. In erysipelas, the area of inflammation is raised above the surrounding skin, and there is a distinct demarcation between involved and normal skin.21
Staphylococcus aureus
S. aureus is currently the most commonly implicated organism in cellulitis following a surgical procedure22 and is also a common cause after nonsurgical wounds. Cellulitis caused by S. aureus is generally more indolent, and fluctulance is more likely to develop. Facial cellulitis in a newborn infant can be a manifestation of staphylococcal osteomyelitis.23
Haemophilus influenzae and Streptococcus pneumoniae
Prior to the introduction of the conjugate vaccine, H. influenzae type b (Hib) was a common cause of cellulitis of the periorbital area and of the cheek (buccal cellulitis) in infants under 2 years of age.24,25 Cellulitis due to Hib results from bacteremic spread and is classically associated with a violacious hue, although occasionally S. pneumoniae can cause a similar pattern.26,27
Groups B, C, and G Streptococci
Group B streptococcal (GBS) infection in the newborn or young infant can occasionally present as focal cellulitis, sometimes with an associated adenitis.28 As with cellulitis caused by Hib and S. pneumoniae, cellulitis in this setting is the result of bacteremic spread. Lumbar puncture is indicated to rule out concomitant GBS meningitis, which may be clinically inapparent.29,30 Other streptococcal groups (e.g., C and G) may occasionally be associated with cellulitis, especially in the extremities of patients with poor venous or lymphatic drainage.31
Erysipelothrix rhusiopathiae
This gram-positive rod is associated with three distinct syndromes, the most common of which is a mild cutaneous infection known as erysipeloid. The diffuse cutaneous form and the septicemic form are much less common.32 Erysipeloid manifests as a sharply demarcated red to purple patch at the site of inoculation, usually the finger or hand. Rare in the United States, the infection is usually acquired occupationally by exposure to contaminated animals, birds, fish, or their products. Penicillin is the drug of choice.
Vibrio Species
Vibrios (especially V. vulnificus, V. alginolyticus, and V. damsela) are uncommon causes of cellulitis. Patients usually have a history of sustaining a wound while having direct contact with seawater or while cleaning shellfish. Patients typically have fever and bullous cellulitis with intense pain at the wound site. Secondary fasciitis or septicemia may ensue, with a mortality rate of 25%.33 Studies in a mouse model as well as in vitro synergy studies suggest that the optimal antibiotic treatment should include both tetracycline and cefotaxime.34,35
Other Organisms
Several other causes (e.g., cutaneous anthrax, sporotrichosis, and Nocardia) may resemble cellulitis initially but usually progress to form ulcerative lesions. They are discussed later in the chapter in the section on skin ulcers.
Laboratory Approach
The white blood cell (WBC) count and differential suggest infection in most patients with an infectious cellulitis, although in mild cases sometimes the WBC count is normal. Blood should usually be obtained for culture before starting therapy, especially for the more severe cases, because once therapy is begun the yield of subsequent cultures falls dramatically. Needle aspiration of an area of cellulitis for Gram stain and culture may be helpful,36 especially if there is a bleb or underlying abscess, elevated periosteum, or joint effusion.
Treatment
The treatment of cellulitis depends on the cause. For most cases of cellulitis, empiric therapy should be directed against Group A streptococcus and S. aureus. For mild cases, a 7–10-day course of an oral antibiotic, such as cephalexin or dicloxacillin is reasonable. A follow-up appointment in 24–48 hours should be made to ensure that the patient is responding to therapy. Cases of cellulitis accompanied by high fever, toxicity, extensive lymphangitis, or rapid progression require hospitalization and intravenous antibiotics. Reasonable choices include oxacillin, cefazolin, or clindamycin. Once the child has improved clinically, a 10-day course can be completed with an oral agent. If the location of the cellulitis suggests a possible underlying cause, the initial antibiotic therapy should be directed at that cause (see Table 17-1). Adjunctive therapy, such as warm compresses and elevation of the affected extremity have not been subjected to rigorous study but probably provide symptomatic relief.
Immunosuppressed Children
Agents that rarely produce cellulitis in normal individuals can cause cellulitis in immunosuppressed children. This is explained, in part, by unusual host susceptibility and, in part, by the frequent prior use of antibiotics in these patients, which selects for altered flora. A vigorous attempt to determine the causative organism should be made. P. aeruginosa and enteric gram-negative organisms are often implicated, and empiric therapy should cover these organisms as well as staphylococci and streptococci. Initial therapy should be given intravenously. Examples of empiric therapy include cefepime or an antistaphylococcal penicillin and an aminoglycoside. Fungi, especially Cryptococcus, as well as mycobacteria, are occasionally implicated.
Mucor and other zygomycetes are primitive fungi that can produce a necrotizing cellulitis resembling necrotizing fasciitis, especially in the newborn period, under adhesive dressings or bandages.37
Noninfectious Cellulitis
Eosinophilic Cellulitis
This self-limited condition usually presents with sudden onset of single or multiple erythematous swellings affecting the extremities or trunk. Histologically, there are diffuse dermal infiltrates of eosinophils, but peripheral eosinophilia is variably present.38 The condition may be familial, and it has been reported in newborns.39 Lesions usually respond to corticosteroids. The term “Wells syndrome” is usually reserved for recurrent cases of eosinophilic cellulitis.
Scleredema
This rare entity is sometimes referred to as “scleredema adultorum” (although it is probably more common in children) or as “scleredema of Buschke.” It is characterized by dermal deposits of mucopolysaccharides, which cause nonpitting induration of the skin, sometimes with erythema. The induration often has a cape-like distribution spreading from the neck and shoulders to the back and trunk. It may occur as a postinfectious phenomenon40,41 or, in adults, as a complication of long-standing diabetes mellitus.42 The disease usually resolves spontaneously over a period of weeks to months.
Erythema Nodosum
Erythema nodosum is often initially mistaken for cellulitis of the legs. The hallmark is tender, bright-red subcutaneous nodules over the tibias, although other areas are occasionally affected. New nodules are round and poorly demarcated, with diameters
from 1–10 cm; over days they become purple and bruise-like (Fig. 17-2). A prodromal period of low-grade fever, arthralgia, and leg pain is common. The erythrocyte sedimentation rate (ESR) is typically elevated, but other laboratory results are usually normal.
from 1–10 cm; over days they become purple and bruise-like (Fig. 17-2). A prodromal period of low-grade fever, arthralgia, and leg pain is common. The erythrocyte sedimentation rate (ESR) is typically elevated, but other laboratory results are usually normal.
Erythema nodosum should be considered a clue that another disease may be present (see Box 17-1). More common in females, it is caused by inflammation in the septae between subcutaneous fat lobules (septal panniculitis) and occurs as an immunologic response to various stimuli.43 In children, infectious triggers are the most common, especially recent group A streptococcal pharyngitis.44,45
In the past, tuberculosis was the infection most commonly associated with erythema nodosum. The lesions can antedate skin test conversion.46 Rarely, the skin test itself, as well as BCG vaccine, is associated with erythema nodosum. Nontuberculous mycobacterial infection may also be associated with erythema nodosum.47 In addition, the nodules caused by infection with Mycobacterium marinum infection in patients with exposure to fish tanks or swimming pools may sometimes resemble erythema nodosum.48
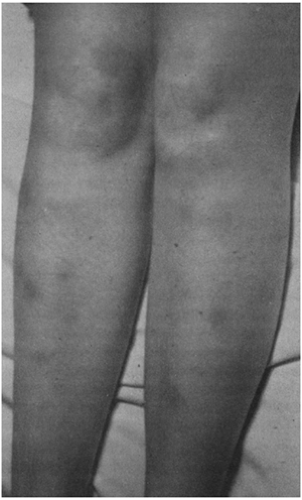 FIGURE 17-2 Erythema nodosum, with red tender nodules. This is sometimes mistaken for cellulitis. (Photo from Dr. Gordon Tuffli.) |
BOX 17-1 Conditions Associated with Erythema Nodosum
| Infectious Group A streptococcus Tuberculosis and nontuberculous mycobacteria Systemic fungal infections (histoplasmosis, coccidioidomycosis, blastomycosis) Epstein-Barr virus Toxoplasmosis Leptospirosis Enteric infections (yersiniosis, salmonellosis, campylobacteriosis) Psittacosis |
| Noninfectious Sarcoidosis Inflammatory bowel disease Malignancy (leukemia, lymphoma) Drug reactions (sulfas, others) Idiopathic |
Other common triggers for erythema nodosum include Epstein-Barr virus (EBV) infection and systemic fungal disease, such as histoplasmosis and coccidioidomycosis.45,49 Some of the more common noninfectious conditions that are associated with erythema nodosum include sarcoidosis, inflammatory bowel disease, Hodgkin’s disease, and use of certain drugs (especially sulfonamides and oral contraceptive agents).43 Other causes of panniculitis, as well as vasculitides, such as polyarteritis nodosa, may sometimes mimic erythema nodosum.43,50
A careful history of possible exposures should be elicited and a thorough physical examination performed. Unless the underlying condition is obvious, a complete blood count, chest roentgenograph, ESR, and a tuberculin skin test should be done. In selected cases, other tests, such as throat culture, antistreptococcal antibodies, EBV titers, and fungal serologies may be indicated.
In children, between one-quarter and one-third of cases of erythema nodosum are idiopathic.44,45 The lesions most often resolve within a few weeks. Usually, only analgesics are necessary for treatment, although severe cases respond to systemic corticosteroids.
Before steroids are prescribed, it is important to exclude an underlying infection that may be worsened by their use.
Before steroids are prescribed, it is important to exclude an underlying infection that may be worsened by their use.
Necrotizing Soft-Tissue Infections
Classification of necrotizing soft-tissue infections may be based on the anatomic structure involved, the infecting organisms, and the clinical manifestations. Because of considerable overlap in these parameters, the nomenclature is confusing. For example, what was once commonly referred to as streptococcal gangrene is now usually called necrotizing fasciitis. However, organisms other than Group A streptococcus may cause necrotizing fasciitis. Some authors define gangrenous infections as those with onset within 24–48 hours after trauma or surgery and necrotizing infections as those with more delayed onset.51
Any classification of necrotizing soft-tissue infections should emphasize that awaiting bacterial classification of the etiology can lead to unnecessary delay and worsened outcome. In one series, operations performed more than 24 hours after recognition of infection resulted in a 70% mortality rate compared with a 36% rate if the time was less than 24 hours.52 An experienced surgeon should be consulted as soon as possible after the recognition of any necrotizing skin infection.
Necrotizing Fasciitis
Guiliano’s classification of necrotizing fasciitis into two types seems to have withstood the test of time.53 Type I is caused by mixed anaerobic and gram-negative aerobic bacilli and usually occurs as a postoperative complication. Type II is caused by Group A streptococcus and most commonly occurs after penetrating injuries, trauma, or during convalescence with varicella. The presentation and initial management of both types are similar.
The affected area is erythematous, warm, shiny, and swollen, with or without bullae, and often with indistinct margins. The appearance is often described as peau d’orange (orange peel) in character. The presence of fever, severe pain out of proportion to the local findings, and systemic toxicity should suggest the possibility of necrotizing fasciitis. Associated laboratory findings include leukocytosis, thrombocytopenia, hyponatremia, hypocalcemia, azotemia, and increased serum creatine phosphokinase.54,55,56 Of patients with streptococcal toxic shock syndrome, about half will have concomitant necrotizing fasciitis.21
In children, systemic toxicity and marked tissue edema may be the only initial clues to the presence of necrotizing fasciitis; fever and leukocytosis are not uniformly present.57 Most infections are either due to Group A streptococcus or are polymicrobial.57,58 In newborns, necrotizing fasciitis can be a complication of omphalitis. Initial periumbilical erythema and swelling can progress rapidly to involve the entire abdominal wall and may progress to involve the flank and chest.59
Fournier Gangrene
Necrotizing fasciitis of the perineal, genital, or perianal regions is referred to as Fournier gangrene.60 This condition is very uncommon in children, with approximately fifty reported cases in the literature.61 In adults, gram-negative bacilli and anaerobes predominate, whereas in children, staphylococcal and streptococcal species are more common. The presentation may be subacute, and the child may not appear systemically ill initially. Management is as for other forms of necrotizing fasciitis.
Clostridial Myonecrosis (Gas Gangrene)
Although historically referred to as “gas gangrene,” clostridial myonecrosis indicates the causal agent and the tissue involved and thus is the preferred term.54 Three major groups are recognized: posttraumatic, postoperative, and spontaneous (which is rare). Clostridial myonecrosis is characterized by crepitation, the crackling feeling consequent to palpation of bubbles of gas under the skin. Crepitant cellulitis should be presumed to be early gas gangrene. The triad of severe pain, tachycardia out to proportion to fever, and crepitus strongly suggests the diagnosis.54 Clostridium perfringens is the usual species recovered in these cases. The course of tissue destruction is usually rapid and severe, with pain, shiny pallor, edema, vesiculation, and crepitus progressing to hemorrhagic darkening and softening of the tissues. Gas may be seen in the subcutaneous tissues on roentgenography. Gram stain of wound discharge or tissue reveals gram-positive rods, usually without neutrophilic infiltration.
Non-Clostridial Gas Gangrene
Necrotizing Cellulitis (Wet Gangrene)
This type is characterized by swollen, boggy tissues, with erythema and blister formation along with tissue destruction. It resembles cellulitis early in its course.
Dry Gangrene
This type is usually secondary to interruption of the blood supply, most often affecting an extremity. The area initially appears dusky, then dark purple, and finally black. If there is no blood vessel disease and an infection is suspected, the diagnosis may be purpura fulminans. The most common cause is meningococcemia,64 which is discussed in Chapters 10 and 11. Neonates with an inherited deficiency of protein C or protein S may present with purpura fulminans.65,66
Meleney’s Synergistic Gangrene
This is caused by co-infection with S. aureus and a microaerophilic streptococcus.67 It is usually a post-surgical complication, especially of the abdomen or chest. It manifests as a slowly expanding ulceration of the superficial fascia. The wound is typically tender and purple.
Compartment Syndromes
Ischemic extremities can result from a compartment syndrome (the compression of a muscle mass within a fascial compartment) as a result of traumatic swelling or vascular occlusion. The syndrome can occur in any age group, including newborns, in whom it can be a consequence of septicemia.68
Diagnosis and Management of Necrotizing Soft-Tissue Infections
Fluid from vesicles or exudate may reveal gram-positive rods (clostridia), gram-positive cocci in pairs and chains (streptococci), or a mixture of organisms in polymicrobial infection. However, caution is indicated, as the Gram stain frequently suggests a single organism when subsequent cultures reveal multiple pathogens.57 In patients with wounds exposed to salt water, Vibrio infection should be considered (see previous section, Cellulitis). Necrotic tissue should be cultured for anaerobes and aerobes. Blood cultures may be positive in up to 50% of cases.59 Imaging studies can sometimes be useful if the diagnosis is in doubt, but negative studies cannot exclude the possibility of necrotizing infection. In clostridial myonecrosis, plain films often show soft-tissue gas dissecting into the muscle. Gas in the tissues is an inconsistent finding in other forms of necrotizing fasciitis. CT may show asymmetric thickening of deep fascia, and MRI may demonstrate abnormal high signal intensity along deep fascial planes on T2-weighted images.69 However, necrotizing fasciitis is a clinical diagnosis, and surgical intervention should not be delayed awaiting imaging studies.
Because most patients with necrotizing fasciitis are in shock, fluid and electrolyte replacement is necessary. Prompt and aggressive surgical debridement of all necrotic tissue is critical. In a series of 20 children with necrotizing fasciitis, all 15 survivors underwent surgical debridement within 3 hours of admission.57 Multiple repeat operations may be required in the first few days.
Initial antibiotic coverage should be broad and include coverage for streptococci, gram-negative bacilli, and anaerobes, even if the initial Gram stain indicates a single organism. The combination of ampicillin, gentamicin, and clindamycin is reasonable empiric therapy. If operative cultures grow only GAS, penicillin plus clindamycin is appropriate. In deep tissue infections with large inocula, streptococcal replication may be slow and penicillin-binding protein expression may be inadequate for a cell-wall active agent, such as penicillin, to kill optimally.70 The addition of a protein synthesis inhibitor, such as clindamycin, may speed killing and decrease toxin production. Penicillin plus clindamycin is also appropriate antibiotic therapy for clostridial myonecrosis.54
The role of nonsteroidal anti-inflammatory drugs (NSAIDS) in the pathogenesis of necrotizing fasciitis is controversial. Some studies have found an association between recent NSAID use and the development of necrotizing fasciitis,71 whereas others have not.72 It is possible that these agents delay the diagnosis of necrotizing fasciitis by masking the symptoms. Given the ready availability of an alternate antipyretic agent, it is probably wise to avoid NSAIDS in children with varicella.73 It is also reasonable to avoid their use in a child with severe cellulitis in whom the possibility of early necrotizing fasciitis is being considered.
The use of intravenous immune globulin (IVIG)
in the treatment of severe, invasive GAS infections has been reported anecdotally74 but not subjected to clinical trials. In a mouse model of group A streptococcal necrotizing fasciitis, the addition of immune globulin did not result in increased bacterial clearance.75 However, given the high mortality rate of necrotizing fasciitis and the biologically plausible consideration that IVIG could neutralize the effect of streptococcal superantigens, its use may be justified.
in the treatment of severe, invasive GAS infections has been reported anecdotally74 but not subjected to clinical trials. In a mouse model of group A streptococcal necrotizing fasciitis, the addition of immune globulin did not result in increased bacterial clearance.75 However, given the high mortality rate of necrotizing fasciitis and the biologically plausible consideration that IVIG could neutralize the effect of streptococcal superantigens, its use may be justified.
The use of hyperbaric oxygen in the treatment of necrotizing fasciitis is likewise controversial. A retrospective study reported a case-fatality rate of 23% among patients receiving hyperbaric oxygen and 66% among those not receiving that therapy.76 Given that the overall case-fatality rate of necrotizing fasciitis in most series is approximately 25%, it is unlikely that the benefit of hyperbaric oxygen is as dramatic as this study would suggest. Its use should not delay surgical intervention.
Traumatic Wound Infections
Traumatic wounds encompass a spectrum from clean lacerations to contaminated compound fractures. This section deals with the prophylaxis and treatment of infections associated with simple lacerations, severe contaminated wounds, penetrating abdominal injuries, and open (compound) fractures. Animal and human bite infections are discussed in Chapter 21.
General Management
Established surgical principles, irrigation, and debridement are the most important factors in the management of traumatic wounds. Antibiotics do not prevent infection in the absence of thorough wound decontamination.77 Saline is the safest and most effective irrigant.78 All devitalized tissue should be carefully debrided. Wounds at low risk for infection can be closed as late as 12–24 hours after the injury, whereas high-risk wounds (contaminated wounds, those in locations with poor blood supply, and those in immunocompromised patients) should be closed within 6 hours.77
TABLE 17-2. INDICATIONS FOR PROPHYLAXIS WITH TETANUS TOXOID AND TETANUS IMMUNE GLOBULIN (TIG) AFTER WOUNDS | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||
A history of tetanus vaccination should be obtained from all persons with traumatic wounds. A guide to the use of tetanus toxoid and tetanus immune globulin is given in Table 17-2.
Follow-up cultures should be done every 2–3 days if drainage is present, to detect the emergence of resistant organisms. Ordinarily, such organisms are present in small numbers and proliferate when the susceptible organisms are inhibited.
Simple Lacerations
The routine use of prophylactic antibiotics in simple, nonbite wound lacerations is not recommended. A meta-analysis of seven randomized, controlled trials demonstrated no difference in the rate of wound infection among those receiving antibiotics as compared with placebo recipients.79
Simple lacerations uncommonly become infected. If signs and symptoms of infection are apparent but no discharge is present to culture, antibiotic therapy directed against S. aureus and group A streptococcus is reasonable, e.g., cephalexin. For infected wounds associated with freshwater exposure, Pseudomonas and Aeromonas are possibilities,
and the use of a fluoroquinolone should be entertained.80 If the infection does not respond promptly to therapy, the incision may need to be reopened and the wound irrigated and debrided.
and the use of a fluoroquinolone should be entertained.80 If the infection does not respond promptly to therapy, the incision may need to be reopened and the wound irrigated and debrided.
Severe Contaminated Wounds
No data exist on the effectiveness of prophylactic antibiotics in this setting, although most experts recommend their use. Using antibiotics in patients with grossly contaminated wounds is probably more appropriately considered empiric therapy than prophylaxis. The appropriate duration of therapy is likewise unknown but is usually about 5–7 days.
In established infection of severe wounds, the responsible organism varies depending on whether or not the patient has received prophylactic antibiotics. Unfortunately, not all reports give those details. In a study in the Vietnam war, severe extremity wounds were cultured on the first, third, and fifth day in a field hospital.81 Bacillus species and S. epidermidis were the most common organisms recovered initially, but Enterobacter, E. coli, Serratia, Klebsiella, and Acinetobacter were also common. Proteus and Pseudomonas were rarely found at first but were commonly recovered from cultures on the fifth day, after antibiotics had been given. A reasonable regimen for empiric therapy in the setting of severe contaminated wounds would be ampicillin-sulbactam or clindamycin plus either gentamicin or ceftazidime.
Open Fractures
Gas gangrene has been reported as an important complication in open forearm fractures of children when antibiotics have not been given.82 Patients with open fractures should receive empiric antimicrobial therapy. Despite the use of antibiotics, the rates of infection are approximately 10–15%.83
It is likely that local factors play a greater role in the risk of infection than antibiotics. In a study of 240 consecutive open fractures of the arm or leg, the most significant risk factors for infection were fracture grade, internal or external fixation, and fracture of the lower leg.84 Timing and duration of antimicrobial therapy did not affect the risk of infection. In a randomized trial, a 24-hour course of antistaphylococcal therapy was as effective as a 5-day regimen.83 A first generation cephalosporin (e.g., cefazolin) can be used for open fractures. Cefazolin does not have activity against Clostridia, however. For heavily contaminated open fractures, coverage against anaerobes and gram-negative organisms should be considered, such as with ampicillin-sulbactam or the combination of clindamycin and gentamicin.
The necessity of antimicrobial prophylaxis in closed fractures is less clear. However, a randomized placebo-controlled trial in 2195 adults with closed extremity fractures found that a single preoperative dose of a cephalosporin reduced the rate of superficial and deep wound infection from 8% down to 4%.85
Penetrating Abdominal Wounds
The use of prophylactic antimicrobials for abdominal injury is predicated on the assumption that hollow visceral injury with resultant bacterial contamination of the intraperitoneal cavity has occurred.86 However, at the time of surgery, definitive evidence of intra-abdominal contamination may be lacking. The use of prophylactic antibiotics in this setting has been demonstrated to be superior to placebo in a randomized controlled trial.87 Injury to the colon carries the highest risk of infection.88 Administration of antibiotics before surgery is associated with a lower incidence of postoperative wound infection and intra-abdominal abscess than initial administration of antibiotics in the intraoperative or postoperative period.89 Antibiotic regimens should cover gut anaerobes, such as Bacteroides fragilis as well as enteric gram-negative rods, such as E. coli. Timing of antibiotic administration (preoperative vs. later) is more important than duration.90 Two studies have demonstrated no difference in infection rates in patients receiving antibiotics for 12–24 hours as compared to 5 days, regardless of degree of injury.91,92
Overall, the incidence of peritonitis and intra-abdominal abscess following abdominal trauma is less than 5%.93 However, the risk increases to greater than 25% in patients with colonic injury, especially if there is concomitant injury to the spleen.94 Bedside ultrasound is sometimes used because of convenience, but computed tomography (CT) is more sensitive in detecting intra-abdominal abcesses.95 Open surgical drainage or, more commonly, CT-guided percutanous drainage of abscesses is usually necessary. The principles of antibiotic therapy in this situation are similar to those for ruptured appendix with peritonitis, as discussed
in Chapter 12. Several options exist, including single-drug regimens (e.g., ampicillin-sulbactam or meropenem) and multiple-drug regimens (e.g., ampicillin, gentamicin, and metronidazole or clindamycin). The initial regimen can then be modified, if necessary, based on results of Gram stain and culture. Duration of therapy is usually 7–14 days.
in Chapter 12. Several options exist, including single-drug regimens (e.g., ampicillin-sulbactam or meropenem) and multiple-drug regimens (e.g., ampicillin, gentamicin, and metronidazole or clindamycin). The initial regimen can then be modified, if necessary, based on results of Gram stain and culture. Duration of therapy is usually 7–14 days.
Burn Infections
Despite remarkable advances in burn care over the past 2 decades, infection remains an important cause of morbidity and mortality in these patients. The increased susceptibility of the burned child to infection relates to impairment in both local and systemic immune response, the loss of the skin barrier, and the frequent need for invasive devices (such as central venous lines, urinary catheters, and endotracheal tubes), which further breach host defenses.
Approximately one-third of infections in burned children involve the burn wound, one-third are secondary to catheter-related bacteremia, and one-third involve other sites (most notably pneumonia and urinary tract infection).96 Depth, type, and extent of the burn are the greatest factors influencing the risk of infection. Age of the child and location of the burn appear to be less important factors.
Among 70 consecutive children cared for in a burn unit, 18 (38%) of 47 of children with full thickness burns developed an infection compared with only 1 (4%) of 23 children with partial thickness burns.96 All nine children who sustained both flame and inhalation injury developed infection as compared with 2 (14%) of 14 children with flame burns that were not accompanied by inhalation injury and eight (19%) of 43 children with scald burns. All 6 children with greater than 30% of the total body surface area burned developed infection; in contrast, infection occurred in 10 (16%) of 64 children with less extensive burns.
Possible Etiologies
Gram-positive organisms (especially S. aureus and S. epidermidis) are the most common cause of infections in burn patients. P. aeruginosa, once the most common cause of infection in this setting, remains the most prominent of the gram-negative pathogens. However, any organism can cause infection in the burn patient. Patients with a history of treatment with systemic antibiotics are at increased risk for late infections with Candida spp., as well as Aspergillus spp. or other filamentous fungi.96,97 Both primary infection and reactivation of herpes viruses may occur, including herpes simplex virus (HSV), varicella-zoster virus (VZV), and cytomegalovirus. Vesicular lesions should raise suspicion of HSV or VZV infection. CMV infection typically presents several weeks after the burn as persistent fever and lymphocytosis.98
Diagnostic Approach
All burn wounds become colonized with bacteria, and distinguishing colonization from infection is critical. The peak incidence of burn wound infection (sometimes referred to as “burn wound sepsis”) is from 6–10 days post burn, but infection can occur at any point during the patient’s course.99 Local signs of burn wound infection include purulence; gray, green, black, or hemorrhagic discoloration; erythema or edema at the wound margin; unexpected eschar separation; conversion of partial thickness to full thickness necrosis; and nonadherence of grafts.96,97 Although the above findings may provide clues to the presence of infection, burn wound biopsy with quantitative cultures demonstrating greater than 105 organisms per gram of tissue in conjunction with histologic evidence of tissue invasion by bacteria is considered the gold standard.97 Systemic signs of infection (tachycardia, fever or hypothermia, leukocytosis or leukopenia, and hypotension) are often present in patients with burn wound infection but may also be present in the severely burned patient in the absence of infection. Blood cultures in burn wound infection are positive in approximately half of cases.
Diagnosis of catheter-associated bacteremia, urinary tract infection, and ventilator-associated pneumonia is similar to that in children without burns. Persistent bacteremia or a new murmur should suggest the possibility of endocarditis.100 Infection with toxin-producing staphylococci or streptococci can lead to toxic shock syndrome.101 Other possible infections include meningitis, suppurative chondritis of ear burns, suppurative thrombophlebitis, osteomyelitis, septic arthritis, and sinusitis consequent to long-term nasotracheal intubation. Multisystem organ failure is the most common cause of death in burn patients and may occur in association with overwhelming infection. However, it can also occur in the clinically uninfected burn patient who has a history of multiple previous infections during
the hospital course. It is postulated that uncontrolled systemic inflammation persists despite control of infection, leading to multiple organ failure.102
the hospital course. It is postulated that uncontrolled systemic inflammation persists despite control of infection, leading to multiple organ failure.102
Treatment
Burn Wound Infection
The penetration of various systemic antibiotics into the burn eschar has not been well-studied, and thus these agents cannot be relied upon as the sole treatment modality.103 Prompt surgical removal of the infected tissue is necessary, as is the use of topical antimicrobial agents. Topical agents are discussed further in the next section.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree