Skeletal complications
Michael A. Via, MD  Ilya Iofin, MD
Ilya Iofin, MD  Jeffrey I. Mechanick, MD
Jeffrey I. Mechanick, MD
Overview
Metabolic bone disease is highly prevalent among cancer patients, owing to the systemic effects of malignancy, the humoral response, and to commonly prescribed cancer therapies. Surgical approaches to acute fracture can drastically impact quality of life. The use of bone antiresorptive agents can prevent fractures in this population and can reduce bone pain in patients with skeletal metastases.
Introduction
Cancer continues to remain a major public health concern as 44% of American men and 38% of women develop cancer at some point in their lifetime. It is estimated that there will be 1,658,370 new cancer cases in the United States in 2015.1 As cancer treatments evolve and survival of patients with cancer continues to improve, skeletal complications of cancer are becoming more prevalent. These complications include pain associated with metastases to bone, fractures, and hypercalcemia of malignancy. The skeleton is the most common site of metastatic disease with ∼70% of patients dying of cancer having skeletal metastases detected on autopsy. A significant portion of these metastases will be clinically relevant and require treatment (see 112, Table 8).2 Once a patient develops skeletal metastases, cure is very unlikely and it must be understood by both the patient and the treating physicians that the goal of treatment is palliation and not cure.2 The five most common cancer types that metastasize to bone are breast, prostate, lung, renal, and thyroid. Almost any cancer subtype can metastasize to bone; however, multiple myeloma is the most common malignancy originating in bone and is responsible for significant skeletal-associated morbidity. As in cases of metastatic carcinoma, cure is unlikely, though systemic control is possible and survival times are improving with evolving therapies. The treatment of problems arising from malignant disease affecting bone requires a multidisciplinary approach involving medical, orthopedic, and radiation oncologists.
Evaluation of a patient with bone lesions
Evaluation of a patient starts with a careful history and physical examination. Location of pain, inciting and alleviating factors, prior history cancer, and of prior treatments are all required to formulate an appropriate treatment plan. In addition to metastases being the potential source of disability, coexisting arthritis and other orthopedic conditions may be the cause of the patient’s pain while the presence of the metastasis may be purely incidental. Imaging of the bone affected by metastatic disease is first done with orthogonal plain radiographs visualizing the entire length bone as the process may be multifocal (Figure 1). Bone lesions may be purely lytic (where only bone destruction is seen, as is typical of lymphoma, multiple myeloma, lung, renal, and thyroid cancer metastases), blastic (where abnormal bone deposition is seen, as is common in metastatic prostate cancer), or mixed (usual for breast cancer metastases). While radioactive technetium bone scans and MRI are very sensitive modalities for detecting metastases, a noncontrast computed tomography (CT) is superior for the evaluation of bone integrity and is often needed to make decisions regarding potential need for surgical intervention. Again, the entire length of the involved bone should be imaged in order to accurately assess fracture risk and help guide potential surgical intervention.
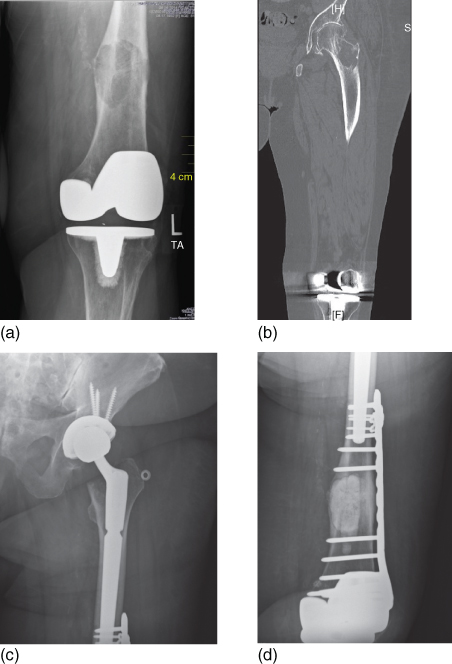
Figure 1 (a) An 81-year-old woman with metastatic breast cancer with a chief complaint of knee pain. Note the destructive lesion in the distal femoral metaphysics above a well-fixed total knee arthroplasty (b) A noncontrast CT of the entire femur was obtained, demonstrating an additional destructive lesion in the femoral neck. While the lesion was asymptomatic, it was at high risk of sustaining a displaced pathologic fracture. Significant degenerative changes are also seen in the hip joint. (c) and (d) The distal femoral lesion was treated with intralesional curettage, internal fixation with a locking plate, and packing with PMMA bone cement. A press-fit long stem total hip arthroplasty was performed to treat the femoral neck lesion and the arthritis. Note the overlap of the two implants that minimize the risk of periprosthetic fracture and protects the entire length of the bone.
Solitary lesions of bone
Special attention needs to be drawn to the patient with a solitary bone lesion but no biopsy-proven metastatic disease. While prior cancer history makes it likely that the newly seen lesion is a metastasis of that cancer, there is no guarantee of that, so a thorough evaluation is required before any intervention. One of the worst errors one could make is to presume that a patient has metastatic disease and failing to recognize a primary bone sarcoma that might be curable. While sarcomas usually affect teenagers and young adults, they can occur in all age groups. The treatment of sarcoma is radically different from treatment of metastatic carcinoma, multiple myeloma, and lymphoma. Bone sarcoma surgery usually entails wide resection of the tumor with curative intent, while most metastatic lesions are treated with intralesional procedures where palliation is the goal. One of the most common orthopedic procedures done for metastatic disease is intramedullary nailing. An intramedullary nail inadvertently placed through a bone sarcoma leads to contamination of the entire length of the bone as well as of the surrounding soft tissues. A patient in whom limb salvage maybe have been possible before intramedullary nailing will require a much more disabling resection at best and a high-level amputation such as a hemipelvectomy at worst. In addition, reaming of the medullary canal pushes sarcoma cells into the systemic circulation and the lungs, making metastatic disease and death more likely.3 Even in the presence of a pathologic fracture through a sarcoma, limb salvage with modern chemotherapy and surgical modalities is possible in most cases if fracture healing can be achieved with cast immobilization or minimally invasive fixation methods that do not lead to tissue contamination.4 Knowledge of the exact tumor diagnosis is required to guide appropriate treatment.
Thus, when presented a patient with an uncertain cancer diagnosis, in addition to evaluation of the affected bone, further staging studies should be undertaken. A CT of the chest, abdomen, and pelvis with oral and IV contrast should be performed to look for visceral metastases and a potential primary tumor. Other skeletal metastases can be detected with a technitium-99 whole body bone scan while detection of a monoclonal spike in serum and/or urine by immunofixation or protein electrophoresis can help establish the diagnosis of multiple myeloma.5 Bone scintigraphy detects new bone deposition in response to tumor activity, trauma, or degenerative joint disease. While it is a sensitive modality for detecting skeletal metastases in most cases, multiple myeloma, metastatic renal cell, and thyroid carcinomas are usually purely lytic in nature where there is little bone deposition. Lesions from these tumors can be missed by bone scintigraphy. When one of these diagnoses is suspected, a skeletal survey (which consists of AP radiographs of the long bones, chest, and pelvis and lateral radiographs of the spine and skull) should be obtained. Positron emission tomography (PET) CT is another sensitive modality for staging, though its exact indications are still being determined. All discovered sites of skeletal involvement should be further evaluated for risk of fracture that may require treatment. Prostate-specific antigen (PSA) levels, usually elevated in patients with metastatic prostate cancer, should be checked in men where this diagnosis is suspected.
This systematic approach will distinguish patients with multiple bone and visceral lesions in whom metastatic disease or multiple myeloma or lymphoma is suspected from the rare patients with solitary bone lesions in whom suspicion of primary bone sarcoma is higher. There are additional benefits to this evaluation algorithm. The most accessible site for biopsy can be found, which can be different from the site of the patient’s original complaint. Metastatic renal cell and thyroid carcinomas are well known to be hypervascular and can hemorrhage significantly during biopsy. Prior knowledge of these diagnoses is useful, as preoperative tumor embolization can be performed and preparations made accordingly.
The final diagnosis, though often suspected based on the radiographic evaluation, is confirmed with a tissue biopsy. If the treating physician is confident of the diagnosis before surgical intervention based on radiographic findings or a known history of metastatic carcinoma or multiple myeloma with confirmatory laboratory findings, an intraoperative frozen section can be used to confirm the diagnosis. Care must be exercised, though, as frozen section is not 100% accurate and provides the correct diagnosis only in 86–94% of biopsies of skeletal lesions.6 If a sarcoma is suspected, then definitive treatment should be delayed until final histologic diagnosis is made. In addition, the reliability of frozen section is dependent on the experience of the pathologist. Most pathologists have limited experience with primary bone tumors. It is important to note that the biopsy tract, especially in cases of an open biopsy, is contaminated by tumor cells and needs to be resected. While that is of little consequence in metastatic disease or multiple myeloma, in cases of sarcoma, inappropriate placement of the biopsy has been shown to adversely affect outcomes, even leading to unnecessary amputations in 4.5% of cases.7 Therefore, biopsy should be performed at a tertiary referral center by, or at least in coordination with, a surgeon with training and expertise in the treatment of bone sarcomas.
Options for treatment of skeletal lesions
A range of treatment options exists for skeletal lesions: it spans the spectrum from observation for asymptomatic lesions that do not present a fracture risk to operative intervention for displaced and impending pathologic fractures. In addition to systemic therapy for the cancer, treatment with bisphophonates or denosumab has been shown to reduce the number of skeletal events, such as compression fractures of the vertebrae and need for surgery or radiotherapy in patients with metastatic bone disease and multiple myeloma.8 Radiation therapy provides pain relief in about 70% of treated lesions. Various therapeutic regimens exist: 8 Gy single-dose treatments have shown similar efficacy to the more traditional and longer 30 Gy 10-day course. Occasionally, the cancer pain recurs, requiring repeat treatment, which is easier to perform with the single-dose treatments.8 Tumors of different histology have different sensitivity to radiation. Multiple myeloma, lymphoma, prostate, and breast cancers are known for their radiosensitivity while renal cell carcinoma is relatively radioresistant.9 Sensitivity to systemic and radiation therapies is one of many factors when choosing most appropriate treatment for a patient with a skeletal lesion. Radiation is most appropriate for lesions that do not present a significant fracture risk. The pelvis, spine, ribs, and scapulae are appropriate sites for radiation treatment in the majority of cases, while lesions of the long bones, especially in the lower extremity, present a higher fracture risk and should be evaluated for potential surgical intervention.
In patients with small lesions that are not structurally significant, where radiation has not provided pain relief, radiofrequency ablation (RFA) and cryoablation are useful minimally invasive treatment options. Under CT guidance, a probe is placed into the lesion to allow local treatment. In RFA, the needle probe delivers a high-frequency alternating current into the surrounding tissues, resulting in heat necrosis. Polymethyl methacrylate (PMMA bone cement) may be injected into the void left by the tumor to provide structural support and additional pain relief.10 Cryoablation is a thermal technique that is conceptually similar to RFA, in which the tumor cells are destroyed not by heating but by cycles of rapid freezing with pressurized argon gas passing through the probe placed in the lesion, followed by slow thawing. Unlike RFA, the zone of thermal necrosis can be visualized on CT. Both modalities can be combined with percutaneous PMMA injection.11 Such image guided thermal ablation techniques are most appropriate for lesions that present minimal risk of fracture, such as vertebral body, sacral and iliac wing lesions, and many acetabular lesions. Once fracture is present or the bone is at significant risk of fracture due to loss of structural integrity, surgery is usually required. Where clinical considerations weight against surgical intervention, particularly in vertebral metastases, PMMA stabilization can relieve pain and restore patient mobility.
Principles of pathologic fracture treatment
The goals of pathologic fracture treatment are to improve function and provide pain relief. The patient and his or her family must be informed and have to understand that the goal of treatment is palliative in nature and will not result in cure. Risk of complications has to be carefully balanced against the potential benefit. Though survival time of cancer patients is difficult to predict, it should exceed the expected surgical recovery time. Pathologic fractures often fail to heal and when they do heal, they are slow to do so. In a series of 123 patients with 129 pathologic fractures, the overall fracture healing rate was only 35%. In patients who survived for more than 6 months, that rate went up to 74%.12 This, and the fact that patients with pathologic fractures have multiple systemic comorbidities, must be kept in mind when selecting the optimal treatment approach. Unlike conventional fractures without an underlying malignancy, where union is likely after a relatively short period of restricted weight bearing, in pathologic fractures, such expectation cannot be made. Fixation must be sufficiently durable to allow nearly immediate almost unrestricted weight bearing and last for the entire lifetime of the patient. Multiple surgeries are not a desirable option. Chemotherapeutic regimens are cytotoxic and are often interrupted to allow wound healing from surgery. As systemic disease can progress during such interruptions of systemic treatment, all efforts should be made to minimize the duration and number of interruptions by minimizing the need for multiple surgeries.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








