Sentinel Lymph Node Biopsy
CRITICAL ELEMENTS
Dynamic Lymphoscintigraphy to Identify the Sentinel Lymph Node-Containing Nodal Basin
Placement of the Sentinel Lymph Node Biopsy Incision
Identification and Removal of One or More Sentinel Lymph Nodes
1. DYNAMIC LYMPHOSCINTIGRAPHY TO IDENTIFY THE SENTINEL LYMPH NODE-CONTAINING NODAL BASIN
Recommendation: Preoperative dynamic lymphoscintigraphy should be performed for accurate identification of one or more lymph node basins containing one or more sentinel lymph nodes.
Type of Data: Observational studies, randomized controlled trials.
Grade of Recommendation: Strong recommendation, moderate-quality evidence.
Rationale
Functionally, the sentinel lymph node (SLN) is defined as the first draining lymph node or group of nodes connected to the primary tumor by an afferent lymphatic channel. From a practical standpoint, the SLN is the lymph node(s) with the highest radioactive counts after dynamic lymphoscintigraphy. Because of the variability of cutaneous lymphatic drainage patterns, dynamic lymphoscintigraphy is necessary to identify cutaneous melanoma SLNs accurately.20,21 Reliance on Sappey’s lines to predict lymphatic drainage patterns for melanoma has been shown to be imprecise and does not account for mapping to multiple nodal basins, interval nodes, or drainage patterns that cross the midline.22,23 Furthermore, dynamic lymphoscintigraphy is helpful in identifying the number of SLNs by delineating single versus multiple afferent
lymphatic channels defining one or more true SLNs (Fig. 9-1).24 The imaging finding of draining lymph nodes in a series along a single lymphatic channel indicates one true sentinel node and tracer migration to second-echelon or nonsentinel nodes (Fig. 9-2). Mapping of lymph nodes in a dynamic fashion, therefore, allows the surgeon to focus on true sentinel nodes and minimizes retrieval of nonsentinel nodes. Information obtained at the time of lymphoscintigraphy may also influence future surgical management by fully defining at-risk nodal basins.
lymphatic channels defining one or more true SLNs (Fig. 9-1).24 The imaging finding of draining lymph nodes in a series along a single lymphatic channel indicates one true sentinel node and tracer migration to second-echelon or nonsentinel nodes (Fig. 9-2). Mapping of lymph nodes in a dynamic fashion, therefore, allows the surgeon to focus on true sentinel nodes and minimizes retrieval of nonsentinel nodes. Information obtained at the time of lymphoscintigraphy may also influence future surgical management by fully defining at-risk nodal basins.
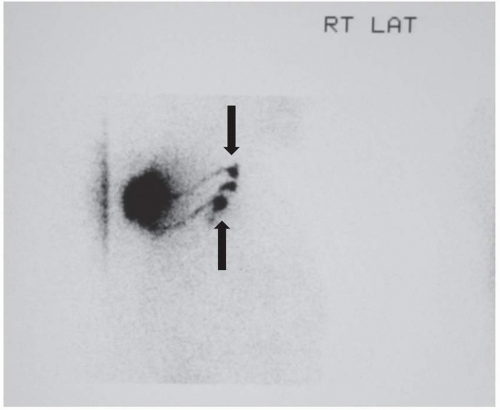 FIGURE 9-1 A lymphoscintigram showing multiple afferent lymphatic channels draining to at least two separate right axillary sentinel lymph nodes (arrows). |
Dynamic lymphoscintigraphy may identify SLNs in “deep” or surgically challenging anatomic locations, such as the internal mammary, iliac, obturator, or periaortic nodal basins. SLN biopsy (SLNB) for deep nodes should be considered only when there is a favorable risk:benefit ratio. Factors to consider when making this decision include the anticipated likelihood of a positive SLN, patient age, comorbidities, obesity, prior operations in the region, and the surgeon’s experience. If the risk:benefit ratio is deemed unfavorable, follow-up of these areas with surveillance imaging may have some benefit.
Preoperative dynamic lymphoscintigraphy can also identify interval SLNs (Fig. 9-3). Interval nodes (sometimes referred to as in-transit, ectopic, or intercalated nodes) are defined as sentinel nodes in other than a named major nodal basin. They are found
in 2% to 9% of patients with melanoma undergoing lymphatic mapping for sentinel node identification, may be the only sentinel node, and may also be the only site of metastatic disease.22,25,26,27,28,29 Careful review of the lymphoscintigraphic imaging is mandatory to avoid overlooking interval sentinel nodes. Interval nodes can be found in the epitrochlear or popliteal area for extremity melanomas and in the auscultatory triangular intermuscular space for truncal melanomas, but they can be found anywhere in the soft tissues along lymphatic drainage pathways. The rate of metastatic disease in interval nodes is comparable to that in standard basins.22,25,26,28,30 Interval sentinel nodes should be removed and evaluated. If an interval node appears to be the only site of drainage on imaging, careful gamma probe evaluation of any major nodal basin in close proximity to the interval node should also be performed.
in 2% to 9% of patients with melanoma undergoing lymphatic mapping for sentinel node identification, may be the only sentinel node, and may also be the only site of metastatic disease.22,25,26,27,28,29 Careful review of the lymphoscintigraphic imaging is mandatory to avoid overlooking interval sentinel nodes. Interval nodes can be found in the epitrochlear or popliteal area for extremity melanomas and in the auscultatory triangular intermuscular space for truncal melanomas, but they can be found anywhere in the soft tissues along lymphatic drainage pathways. The rate of metastatic disease in interval nodes is comparable to that in standard basins.22,25,26,28,30 Interval sentinel nodes should be removed and evaluated. If an interval node appears to be the only site of drainage on imaging, careful gamma probe evaluation of any major nodal basin in close proximity to the interval node should also be performed.
 FIGURE 9-2 A lymphoscintigram showing drainage to a superficial left groin sentinel lymph node (arrow) followed by secondary drainage to nonsentinel nodes within the inguinal and iliac region. |
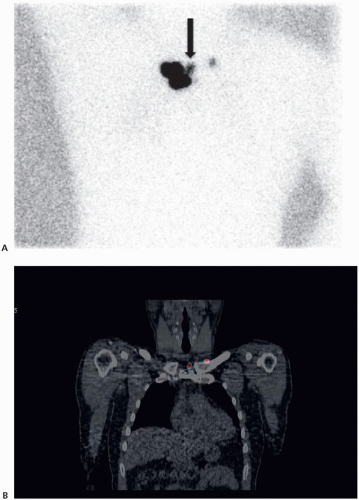
SLNB is technically more challenging for head and neck melanomas due to complex drainage patterns, increased number and small size of lymph nodes, and the frequent close proximity of the nodes to the primary tumor injection site.31,32,33 Consequently, false-negative SLNBs are more common for the head and neck region than for other anatomic sites.34,35 The use of single-photon emission computed tomography combined with computed tomography (SPECT/CT) facilitates identification and
subsequent removal of SLNs for head and neck melanoma.36,37,38 A theoretical advantage of SPECT/CT is better anatomic localization in three dimensions than that provided by the planar views of standard lymphoscintigraphy (Fig. 9-4).39,40 Accordingly, it has been reported that SPECT/CT has higher sensitivity, better detection of sentinel nodes in close proximity to the injection site, and improved localization as compared to standard lymphoscintigraphy.41
subsequent removal of SLNs for head and neck melanoma.36,37,38 A theoretical advantage of SPECT/CT is better anatomic localization in three dimensions than that provided by the planar views of standard lymphoscintigraphy (Fig. 9-4).39,40 Accordingly, it has been reported that SPECT/CT has higher sensitivity, better detection of sentinel nodes in close proximity to the injection site, and improved localization as compared to standard lymphoscintigraphy.41
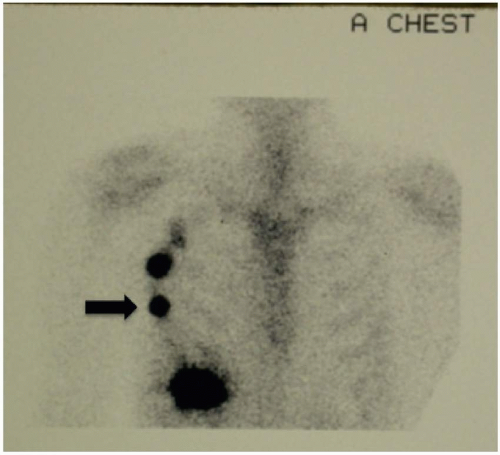 FIGURE 9-3 A lymphoscintigram showing a right chest interval sentinel lymph node (arrow) followed by secondary drainage to a right axillary nonsentinel node. |
These characteristics of SPECT/CT may be particularly advantageous for SLN mapping for head and neck melanomas and in other challenging situations.37,42 However, because of the added cost, higher radiation dose, and lack of a clear outcome benefit, SPECT/CT should be used judiciously for other locations.
Technical Aspects
Lymphoscintigraphy for cutaneous melanoma involves injection of a radioactive tracer in the form of a radiocolloid at the primary tumor or biopsy site. In the United States, technetium Tc 99m is combined with sulfur colloid and, because of its size
properties (<200 nm), is taken up into the afferent lymphatics and eventual draining lymph nodes.25 Although other radioactive agents are used in different countries, technetium Tc 99m has the advantage of being short-lived (with a biologic half-life of 24 hours in humans) and almost pure gamma emission decay that makes it readily detectable by a gamma camera and less harmful to exposed tissue than other radioactive tracers.43 In March 2013 the US Food and Drug Administration (FDA) also approved technetium Tc 99m tilmanocept for SLN mapping for melanoma and breast cancer. This radiocolloid appears to be as effective as vital blue dyes in identifying SLNs, but there are no data comparing its efficacy with that of other radiocolloids.
properties (<200 nm), is taken up into the afferent lymphatics and eventual draining lymph nodes.25 Although other radioactive agents are used in different countries, technetium Tc 99m has the advantage of being short-lived (with a biologic half-life of 24 hours in humans) and almost pure gamma emission decay that makes it readily detectable by a gamma camera and less harmful to exposed tissue than other radioactive tracers.43 In March 2013 the US Food and Drug Administration (FDA) also approved technetium Tc 99m tilmanocept for SLN mapping for melanoma and breast cancer. This radiocolloid appears to be as effective as vital blue dyes in identifying SLNs, but there are no data comparing its efficacy with that of other radiocolloids.
At the peritumoral site of the melanoma or the excision scar, aliquots of 0.1 to 0.5 mCi of technetium Tc 99m are injected intradermally in each of four quadrants. The accuracy of SLN identification by lymphoscintigraphy appears to be equivalent whether the in situ primary tumor or a biopsy or excision scar is injected.26 Images are typically acquired continuously for the first 10 minutes to visualize flow from the injection site. After this continuous detection, images are then acquired every 3 minutes, and views are taken to localize the draining lymph nodes and rule out any other potential drainage sites. In the rare case that a lymph node is not detected at 1 hour, imaging continues for another 30 minutes prior to concluding that the SLN failed to map. Mapping failures are uncommon, generally reported as <2%.44,45,46
2. PLACEMENT OF THE SENTINEL LYMPH NODE BIOPSY INCISION
Recommendation: Sentinel lymph node biopsy incision placement should be determined primarily by a synthesis of the following elements: review of the preoperative dynamic lymphoscintigraphy, the area of greatest radioactivity within the nodal basin or interval node site identified by the gamma probe, and the potential need for completion lymphadenectomy. Secondary considerations include cosmesis and functionality. When feasible, an attempt should be made to place the biopsy incision at the location of any anticipated completion lymph node dissection incision.
Type of Data: Observational studies.
Grade of Recommendation: Weak recommendation, high-quality evidence.
Rationale
Ideally, the sentinel lymph node biopsy (SLNB) incision should be small and directed by the point of greatest radioactivity with the localized nodal basin or interval node site.47,48,49 In addition, incision placement should also take into consideration possible future surgery for completion lymph node dissection (CLND) and the minimization of cosmetic and functional impairment.38,42
3. IDENTIFICATION AND REMOVAL OF ONE OR MORE SENTINEL LYMPH NODES
Recommendation: The one or more nodes defined as the sentinel lymph node(s) are those with the highest radioactive counts after dynamic lymphoscintigraphy and
any additional lymph nodes with >10% of the maximal ex vivo radioactive counts of the “hottest” sentinel node (the one with the highest radioactivity). The injection of a vital blue dye can assist in the localization of the sentinel lymph node but is considered optional. Blue dye should not be used in pregnant women.
any additional lymph nodes with >10% of the maximal ex vivo radioactive counts of the “hottest” sentinel node (the one with the highest radioactivity). The injection of a vital blue dye can assist in the localization of the sentinel lymph node but is considered optional. Blue dye should not be used in pregnant women.
Type of Data: Observational studies and randomized controlled trials.
Grade of Recommendation: Strong recommendation, moderate-quality evidence.
Rationale
Within nodal basins with a somewhat linear arrangement of the lymph nodes, such as the groin, dynamic lymphoscintigraphy can frequently discern between the sentinel lymph node (SLN) and any secondary lymphatic drainage to second-echelon nonsentinel nodes along the same lymphatic channel. However, in nodal basins with a more three-dimensional configuration, such as those of the axilla and the neck, it can sometimes be difficult to determine whether there are multiple SLNs from separate lymphatic channels or if additional radioactive lymph nodes are simply secondary drainage. Consequently, the most widely accepted standard is to also consider as SLNs any additional lymph nodes with radioactivity counts >10% of maximal ex vivo radioactivity counts of the hottest SLN.48,50 In an attempt to minimize unnecessary removal of second- and third-echelon lymph nodes, which are not true SLNs, some investigators have used higher thresholds, such as removal of nodes with >20% to 70% of the counts of the hottest node.51,52 However, the 10% rule has proven to be efficacious in terms of identifying lymph nodes that should or should not be removed, as well as controlling the costs associated with the surgical procedure and pathologic processing.53,54
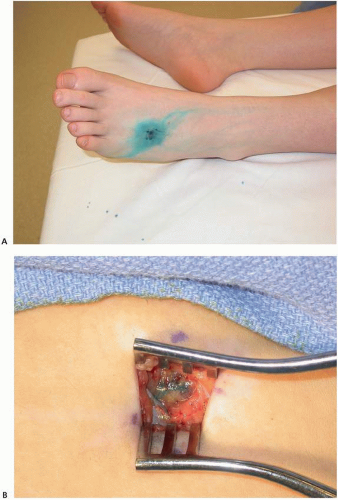
The original reports of melanoma SLNB technique included the injection of patent blue V, or isosulfan blue, at the tumor site with subsequent intraoperative observation of a blue-stained lymphatic leading to the sentinel node (Fig. 9-5).55 It is typically injected intradermally around the primary melanoma or biopsy or excision scar in the operating room at the start of the procedure. If blue dye is used, it is in combination with radioactive-tracing dynamic lymphoscintigraphy to identify the appropriate nodal basin preoperatively and the specific SLN(s) intraoperatively. The use of blue dye alone is associated with reported SLN detection rates of approximately 85%.56,57 When combined with radioactive tracer, detection rates for this dual technique exceed 99%.57
The use of isosulfan blue dye has been associated with rare anaphylactic reactions and reports of persistent skin staining (injection site “tattooing”). Both blue dyes are labeled as FDA Pregnancy Category C, whereas technetium Tc 99m at standard mapping doses is below the threshold of concern in pregnant women.58,59,60
Data comparing the combined use of blue dye and radioactive tracer suggest that blue dye identifies nonradioactive SLNs in <2% of cases.61 Because SLN identification with radioactive tracer alone is very high, many surgeons have omitted the use of vital blue dyes. This has not been associated with an increased rate of missed SLNs.62,63 For these reasons, the use of a vital blue dye at the time of SLNB could be considered optional by experienced surgeons.
Technical Aspects
Ideally, the interval between radioactive tracer injection for dynamic lymphoscintigraphy and operative SLNB should be minimized. This identification is typically performed on the same day as surgery, but it can be up to 12 to 18 hours earlier to facilitate surgical scheduling. However, the greater the time interval between the initial radioactive tracer injection and the SLNB surgery, the higher the likelihood of radioactive tracer migration to second-echelon non-SLNs (NSNs) and beyond, potentially leading to removal of an increased number of radioactive lymph nodes that do not represent true SLNs.
Anatomically, the primary tumor site can occasionally be in close proximity to the SLN-containing nodal basin. In this case, the injection site radioactivity from the radioactive tracer injection can potentially mask an underlying SLN, which can be missed on dynamic lymphoscintigraphy. An example would be a melanoma overlying the proximal back region where the high levels of injection site radioactivity obscure an underlying supraclavicular SLN. In this situation, the underlying lymph node basin should always be examined with the handheld gamma probe after the wide local excision (WLE) has been performed to identify any SLNs that may have been undetected on dynamic lymphoscintigraphy. A similar scenario is when the primary tumor site is directly in line with the direction that the gamma probe will be utilized while examining the lymph node basin during the SLNB procedure. The high levels of the primary tumor injection site radioactivity can spuriously elevate the background nodal basin radioactive counts, a phenomenon referred to as “shinethrough.”47 This radioactivity interference can be mitigated by performing the WLE of the primary tumor site (to remove the skin source of radioactivity) prior to attempting the SLNB.38,42,47
At the time of the surgical procedure, the nodal basin containing the SLN(s) is examined with the handheld gamma probe to identify the site of maximal radioactivity. This is often marked on the skin with a surgical marker. As noted previously, the incision that would be utilized to perform any potential future completion lymphadenectomy should be considered when determining the SLNB incision location. After creation of the incision, the gamma probe is used to identify the lymph node with the highest radioactive count (Fig. 9-6). If blue dye is injected, visual inspection for a blue-stained lymphatic and blue lymph node is also performed. After removal of the hottest SLN, ex vivo radioactive counts are obtained. The nodal basin is further examined with the gamma probe, and any additional lymph nodes with radioactive counts >10% of the hottest SLN ex vivo counts are also removed and sent to pathology as additional SLNs. All of the SLNs have been identified once the residual nodal basin radioactive counts are <10% of the hottest SLN ex vivo counts. Again, if blue dye is utilized, a blue, but nonradioactive lymph node should also be considered an SLN and removed.
SLN identification rates have been found to be up to 99.4% when the procedure is performed by a trained multidisciplinary team.44,45 If, despite the use of the SLNB techniques described here, no SLN is identified, termination of the SLNB procedure and close clinical follow-up is generally appropriate.
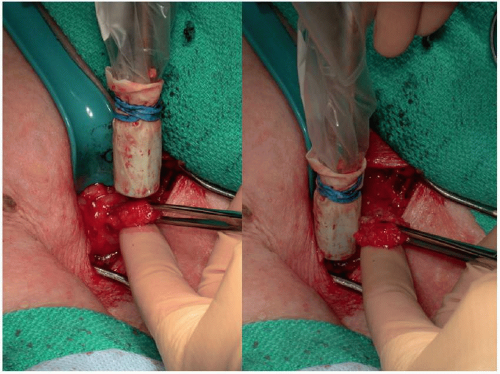 FIGURE 9-6 Intraoperative utilization of a gamma probe to identify the radioactive sentinel lymph node. |
Occasionally, a lymph node that contains a significant amount of metastatic tumor may not become radioactive on dynamic lymphoscintigraphy. Therefore, any lymph node clinically suspicious by palpation or visual inspection of the nodal basin at the time of the SLNB procedure should also be removed.
Accurate histopathologic examination of melanoma SLNs is critical for staging and treatment planning. The gold standard is permanent evaluation with step sectioning and immunohistochemistry. In the absence of this rigorous pathologic assessment, retrospective data suggest that overlooked tumor in the SLN, rather than other sources of technical failure, is responsible for up to 80% of same-basin nodal recurrences.64 Therefore, preserving the SLN intact is important for accurate formal pathologic assessment. Although intraoperative frozen-section analysis is technically possible, accuracy is low for small-volume nodal disease, and tissue loss during frozen-section processing might lead to missed metastases and understaging.65 Intraoperative touch preparation analysis would mitigate specimen loss, but it also has a limited ability to detect micrometastatic disease. For these reasons, intraoperative pathologic assessment of the SLN should not be routinely performed. It should be reserved for situations in which an intraoperative diagnosis of nodal metastatic disease would dramatically alter the remainder of the planned operative procedure.
Key Question: Sentinel Lymph Node Biopsy
In what subsets of patients with melanoma is sentinel lymph node biopsy controversial?
INTRODUCTION
The use of lymphoscintigraphy to define the lymphatic drainage from a primary cutaneous melanoma was introduced in the 1970s.66 Morton et al55,67 then pioneered the clinical technique of using lymphatic mapping to identify the first or “sentinel” lymph node to determine the pathologic status of the regional lymph node basin in the absence of clinical nodal metastatic disease. After the dissemination of the sentinel lymph node biopsy (SLNB) technique throughout the world, a fundamental question became “What are the benefits of performing SLNB?”
The Multicenter Selective Lymphadenectomy Trial (MSLT-I) was a multi-institutional, prospective, randomized study of 2,001 patients with melanomas classified as being of intermediate thickness (1.2 to 3.5 mm) or thick (>3.5 mm). It compared wide excision and SLNB followed by immediate completion lymph node dissection (CLND) for a positive SLNB (SLNB arm) to wide excision alone with nodal basin observation, reserving lymph node dissection (LND) for clinically detected nodal metastatic disease (observation arm).45 The results of this trial identified three potential benefits of SLNB. The first benefit is prognostication. In intermediate-thickness melanoma, the 10-year melanoma-specific survival (MSS) was 85.1% if the SLNB was negative but only 62.1% for a positive SLNB (P <0.001). The prognostic value of the SLNB status was also significant for thick melanomas: a 64.6% 10-year MSS for a negative SLNB versus 48.0% for a positive SLNB (P = 0.03). As compared to all other traditional melanoma variables (Breslow thickness, ulceration, anatomic location, sex, age, and Clark level), SLNB status was the most powerful predictor of melanoma disease recurrence and death on multivariate analysis.
The total number of positive lymph nodes, size, and the presence of extracapsular extension are all features associated with an increased risk of regional lymph node basin relapse after CLND. Therefore, a second potential benefit of performing SLNB is improved nodal basin control. In MSLT-I, the median number of positive lymph nodes in the SLNB positive patients who underwent CLND was 1.4.45 The total number of positive lymph nodes was one in 70.5% of patients and four or more in only 1.6%. In contrast, the median number of positive nodes at the time of clinical relapse for patients in the observation arm was 3.3. One node was positive in 39.5%, and four or more were positive in 27.7%. In two large prospective studies, the rate of regional nodal basin recurrence after CLND for a positive SLNB was 4.2% to 4.9%.68,69 A third potential benefit of SLNB is improved survival through the early detection of occult nodal metastatic disease. After 10 years of follow-up for MSLT-I, there was no statistically significant difference in 10-year MSS between the SLNB and observation arms for both intermediate-thickness melanoma (81.4% vs. 78.3%, P = 0.18) and
thick melanoma (58.9% vs. 64.4%, P = 0.56).45 A subset analysis of lymph nodepositive intermediate-thickness melanoma in the SLNB versus observation arms did show a significantly improved 10-year MSS for the SLNB patients (62.1% vs. 41.5%, P = 0.006), but there was no significant difference in the thick primary cohort (48% vs. 45.8%, P = 0.78).
thick melanoma (58.9% vs. 64.4%, P = 0.56).45 A subset analysis of lymph nodepositive intermediate-thickness melanoma in the SLNB versus observation arms did show a significantly improved 10-year MSS for the SLNB patients (62.1% vs. 41.5%, P = 0.006), but there was no significant difference in the thick primary cohort (48% vs. 45.8%, P = 0.78).
Multiple studies have shown that the risk of a positive SLNB directly correlates with increasing Breslow thickness. Although SLNB is associated with low morbidity, it typically requires general anesthesia. Therefore, the decision to perform an SLNB in a particular patient should take into account the likelihood of occult nodal metastatic disease, the risks of the procedure, and the benefits (prognostic value, therapy, regional control, and possibly survival).
Identifying patients with a positive SLNB results in more uniform high-risk melanoma cohorts for adjuvant therapy or clinical trial participation. In contrast, followup strategies can be tailored based on the favorable survival profile for a patient with a negative SLNB. Cancer treatment guidelines have attempted to define the patients who should undergo SLNB.38 A more nuanced understanding of the data can help to answer the question, “In what subsets of patients with melanoma is SLNB sometimes controversial?”
METHODOLOGY
An organized search of PubMed was performed with the assistance of a library informationist on December 9, 2015. Keyword combinations included the following medical subject heading (MeSH) terms (mh), title/abstract words (tiab), and subject headings (sh). Terms longer than a single word or abbreviation were enclosed within quotation marks and followed without a space by the keyword abbreviation, which was enclosed in brackets. The Boolean search operators “or” and “and” appeared in all capital letters, and parentheses were used to combine search terms: (melanoma [mh] or melanoma [tiab]) and (sentinel lymph node biopsy [mh] or sentinel lymph node biopsy [tiab] or sentinel lymph node biopsies [tiab] or sentinel lymph node [tiab] or sentinel lymph nodes [tiab] or sentinel biopsy [tiab] or sentinel biopsies [tiab] or sentinel node biopsy [tiab] or sentinel node biopsies [tiab] or sentinel lymphadenectomy [tiab] or sentinel lymphadenectomies [tiab] or slnb [tiab]) and (surgical procedures, operative [mh] or surgery [sh] or surgery [tiab] or surgeries [tiab] or surgical [tiab] or surgically [tiab] or surgeon [tiab] or surgeons [tiab] or operation [tiab] or operations [tiab]).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree