Introduction
INTRODUCTION
As the sixth most common cancer and one affecting most age groups, melanoma remains a clinical and epidemiologic problem. In 2018 in the United States, the estimated number of new annual cases will be 91,270 and new annual deaths will be 9,320.1 Risk factors include a fair complexion and exposure to ultraviolet radiation, both natural sunlight and indoor tanning. Melanoma is slightly more common in males, who have a lifetime risk of 1 in 33 as opposed to that in females of 1 in 52. Although the incidence of melanoma has been increasing over several decades, mortality rates have flattened.2 This suggests that improved physician awareness and patient education might result in earlier detection. At the time of diagnosis, 84% of patients with melanoma will present with localized disease, 9% with regional lymph node involvement, and 4% with distant metastases.1 Corresponding 5-year relative survival rates are 98%, 62%, and 18%, respectively.
Surgical wide excision, including the underlying local lymphatics at risk of micrometastases, is the potentially curative treatment for the primary melanoma. In most cases, surgical therapy is the only treatment required. Margin width recommendations for wide excision date back to those set in 1907 when W. S. Handley3 described in one autopsy report multiple lymphatic tumor nodules around the primary melanoma. His suggestion for solving this problem was to resect 1″ (2.5 cm) of skin surrounding the primary tumor with 2″ (5 cm) of subcutaneous tissue as well as the fascia and underlying muscle. This anecdotal observation led to the standard-of-care 5-cm excision margin for the next half-century, which typically mandated a skin graft. Multiple randomized trials examining margin width have subsequently been performed to address this issue, primarily for intermediate thickness melanoma.4,5,6,7,8,9 The collective results of these studies are that wide excision margins have a nominal impact on locoregional recurrence and no effect on survival (except for one recent study). From a patient perspective, wider margins are associated with an increase in postoperative morbidity.
Surgical management of melanoma was revolutionized in the 1990s by Donald Morton, who introduced sentinel lymph node biopsy.10 Previously, the at-risk regional nodal basin was either observed over time (an approach that did not provide prognostic or staging information) or was subjected to an elective lymph node dissection (which had the attendant risk of morbidity, a particular concern, given the possibility of negative lymph nodes). The scientific rationale for sentinel node biopsy was that (1) lymphatic drainage is orderly and predictable, with the sentinel lymph node accurately predicting the risk of nodal involvement for the remainder of the nodal basin; (2) selectively removing a limited number of lymph nodes is associated with less morbidity than a formal lymphadenectomy; (3) the presence or absence of nodal metastatic melanoma is prognostic for survival; and (4) early removal of clinically occult micrometastatic nodal disease might improve survival. After 20 years of widespread sentinel node biopsy implementation, the majority of these premises have been validated. A large meta-analysis of melanoma sentinel lymph node biopsy showed that a sentinel node could be identified in more than 98% of patients, the false-negative rate was 12.5%, and the estimated risk for nodal basin recurrence
after a negative sentinel node biopsy was ≤5%.11 Reported complication rates for sentinel node biopsy are low: seroma, 5.1%; infection, 2.9%; lymphedema, 1.3%; hematoma, 0.5%; and nerve injury, 0.3%.12 The sentinel node status has been shown to be the most powerful predictor of disease recurrence and death from melanoma of all traditional prognostic variables.13,14 Finally, in a subset analysis of data from the Multicenter Selective Lymphadenectomy Trial (MSLT-I), patients with a melanoma of intermediate thickness (1.2 to 3.5 mm) and a positive sentinel lymph node did have a significantly better 10-year melanoma-specific survival rate than those in the observation arm who developed clinical nodal metastatic disease (62.1% vs. 41.5%, P = 0.006).13 This survival benefit was not seen in patients with positive sentinel nodes who had thick primary melanomas.
after a negative sentinel node biopsy was ≤5%.11 Reported complication rates for sentinel node biopsy are low: seroma, 5.1%; infection, 2.9%; lymphedema, 1.3%; hematoma, 0.5%; and nerve injury, 0.3%.12 The sentinel node status has been shown to be the most powerful predictor of disease recurrence and death from melanoma of all traditional prognostic variables.13,14 Finally, in a subset analysis of data from the Multicenter Selective Lymphadenectomy Trial (MSLT-I), patients with a melanoma of intermediate thickness (1.2 to 3.5 mm) and a positive sentinel lymph node did have a significantly better 10-year melanoma-specific survival rate than those in the observation arm who developed clinical nodal metastatic disease (62.1% vs. 41.5%, P = 0.006).13 This survival benefit was not seen in patients with positive sentinel nodes who had thick primary melanomas.
CLINICAL STAGING
For melanoma, the primary tumor features important for accurate staging and prognostication are Breslow depth (in millimeters), tumor ulceration, mitotic rate, and microsatellites. All of these variables should be addressed by the biopsy pathology report. The presence or absence of regional lymph node metastases is determined by clinical examination and sentinel lymph node biopsy, if clinically appropriate. Distant metastatic disease is assessed by history, physical examination, and the selective use of adjunctive imaging.
Routine use of staging imaging in all newly diagnosed patients is not supported by the current literature. Even in patients with positive sentinel lymph nodes, in whom one would expect a higher risk of harboring occult distant metastatic disease, the yield of routine staging imaging is low. Aloia et al15 reviewed 270 patients with melanoma and no clinical concern for distant metastatic disease who underwent routine staging with computed tomography (CT) scan and/or magnetic resonance imaging after a positive sentinel node biopsy. Imaging studies led to suspicion of synchronous distant metastatic disease in 8.6% of patients, but further workup, including biopsy, to confirm distant metastases was ultimately positive in only 1.9%. The authors’ conclusion was that asymptomatic patients with newly diagnosed nodal metastatic melanoma by sentinel node biopsy do not require additional imaging prior to proceeding with completion lymph node dissection.
In patients with clinical nodal metastatic disease or resectable distant metastases, several studies have shown that the results of a staging positron emission tomography (PET) scan or a PET scan combined with a CT scan (PET-CT) can alter the proposed surgical plan in 15% to 35% of cases.16 False-positive rates were significant, which could lead to an incorrect change in disease management. A meta-analysis of conventional imaging modalities for melanoma staging and surveillance found that ultrasonography had the highest sensitivity and specificity for regional lymph node assessment, whereas PET-CT was better for detecting distant metastases.17 Similar to other studies, this study found PET-CT had a high number of overall false-positive results and had a very low positive predictive value (33%) in patients at lower risk of metastases than others. In conclusion, staging imaging should be pursued in patients with clinical findings suggestive of nodal or distant metastatic disease (e.g., clinically palpable regional
lymph nodes). Imaging findings suspicious, but not absolutely conclusive, for distant metastatic disease should be confirmed pathologically with a biopsy.
lymph nodes). Imaging findings suspicious, but not absolutely conclusive, for distant metastatic disease should be confirmed pathologically with a biopsy.
Several changes to the new eighth edition of the melanoma staging manual by the American Joint Committee on Cancer are relevant to surgical management of melanoma.18 Breslow depth will now be rounded to the nearest tenth of a millimeter, not the nearest hundredth of a millimeter. Although mitotic rate still has some prognostic value, it is no longer incorporated into the staging of primary melanomas ≤1.0 mm. The T1 category has also been subcategorized by tumor thickness to include a 0.8-mm threshold. Consequently, T1 has been subdivided and redefined as T1a, that is, <0.8 mm without ulceration, and T1b, that is, <0.8 mm with ulceration or 0.8 to 1.0 mm with or without ulceration. In the previous edition, nodal metastatic disease was defined as microscopic versus macroscopic. Given that these designations suggested the method of detection, nodal disease subcategories a and b have been redefined as “clinically occult” (detected by sentinel node biopsy) and “clinically detected,” respectively. The new eighth-edition American Joint Committee on Cancer melanoma staging manual tumor-node-metastasis system is summarized in Table I-1.
MULTIDISCIPLINARY CARE
The majority of patients who undergo surgery for primary melanoma will not require additional therapy. Cases characterized by bulky clinical nodal metastatic disease or at high risk of harboring occult distant metastases are two clinical types for which multidisciplinary adjuvant therapy might be considered. Unlike those in breast cancer, lymph node metastases in melanoma can become very large and can be associated with matting and extracapsular extension into adjacent critical neurovascular structures. Therefore, one of the purported benefits of melanoma sentinel lymph node biopsy is the early detection and treatment of low-volume nodal micrometastatic disease. Regional recurrence after completion of lymph node dissection for a positive sentinel node in large prospective studies has been <5%.19,20
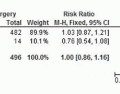
Unfortunately, some patients still present with newly diagnosed bulky clinical lymph node metastases for whom therapeutic lymphadenectomy alone is unlikely to eradicate all nodal basin disease completely. Risk factors for nodal basin recurrence after a lymph node dissection for nodal metastatic melanoma include the total number of positive lymph nodes, the size of the positive lymph nodes, the extent of extracapsular extension, and the anatomic nodal basin, especially the cervical basin.21,22 Several retrospective studies have examined the benefit of adjuvant nodal basin radiation on regional control for nodal disease at high risk of recurrence. Single-arm adjuvant radiation studies have reported regional control rates of 74% to 94%.23,24,25 Retrospective studies comparing therapeutic lymphadenectomy with or without adjuvant radiation have shown mixed results for regional control.26,27,28,29,30,31 A large retrospective two-institution study of adjuvant radiation for high-risk nodal metastatic disease found a statistically significant improvement in 5-year regional control rates for therapeutic lymphadenectomy with adjuvant radiation over therapeutic lymphadenectomy alone (87% vs. 52%, P <0.0001).31 There is also one prospective, randomized study of therapeutic lymphadenectomy for high-risk, clinical nodal metastatic melanoma (ANZMTG 01.02/TROG 02.01) with 48 Gy of adjuvant nodal
basin radiation compared with therapeutic lymphadenectomy alone.32 With a median follow-up of 73 months, the adjuvant radiation group had a significantly lower rate of nodal basin relapse than did the group with surgery alone (21% vs. 36%, P = 0.023); however, there was no difference in overall survival.
basin radiation compared with therapeutic lymphadenectomy alone.32 With a median follow-up of 73 months, the adjuvant radiation group had a significantly lower rate of nodal basin relapse than did the group with surgery alone (21% vs. 36%, P = 0.023); however, there was no difference in overall survival.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree