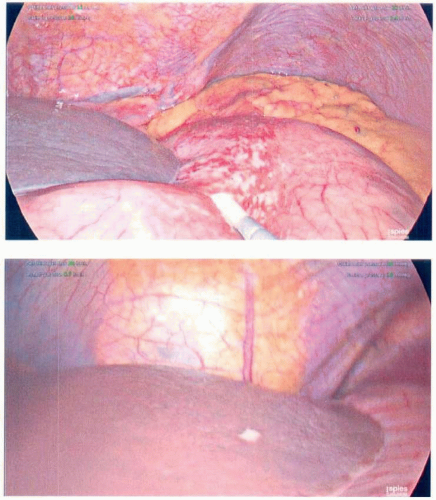
Intraoperative Staging
Resection of Primary Tumor
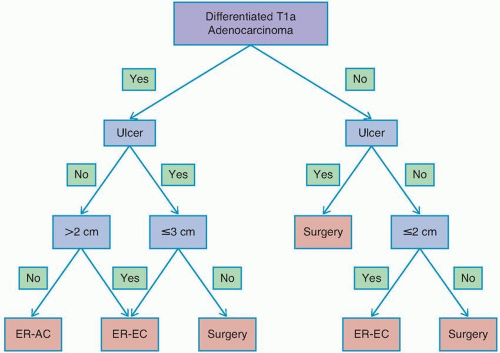
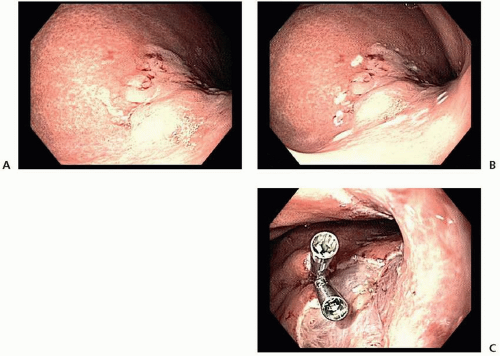
Endoscopic Resection
Partial and Total Gastrectomy
Minimally Invasive Gastrectomy
Assessment of Surgical Margins
Regional Lymphadenectomy
Reconstruction of the Gastrointestinal Tract
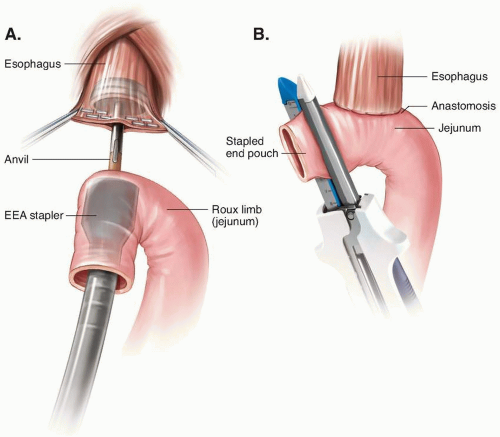
After Total Gastrectomy
After Distal and Subtotal Gastrectomy
After Proximal Gastrectomy
Placement of Drains and Tubes
Placement of Intraperitoneal Drain
Placement of Small-Bowel Feeding Tube
can also be performed after the administration of preoperative systemic therapy to measure the therapeutic response and to confirm the absence of occult metastatic disease prior to gastric cancer surgery. And at the same time, other adjunct procedures can be performed, such as placement of a feeding jejunostomy tube in advance of neoadjuvant therapy or placement of a central line catheter for systemic therapy.8,9,10
TABLE 3-1 Incidence of Occult Metastases at the Time of Diagnostic Laparoscopy for Patients Eligible for Curative Gastric Resection on the Basis of Preoperative Imaging Studies | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
the management of gastric cancer. A recent analysis of national data showed that diagnostic laparoscopy was performed in only 8% of a population of older patients with gastric cancer.11
be spared an unnecessary laparotomy. Thus, sequential laparoscopic procedures and peritoneal sampling may have a role in specific scenarios, such as selecting patients for a potentially curative resection after neoadjuvant therapy and sparing those for whom the gastric resection would be futile. As noted, cytologic analysis of the samples acquired at peritoneal lavage may require several days; therefore, preoperative therapy or gastrectomy may have to be suspended until results are received.
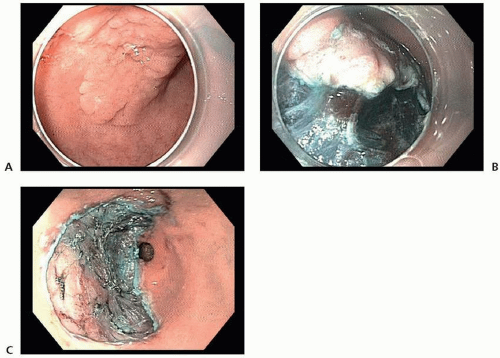
Several promising reports have described short-term outcomes after endoscopic resection of EGC at Western centers, with curative resection rates in excess of 80%.29,30 However, recent epidemiologic data have indicated significant racial and ethnic disparities in the risk of lymph node metastasis in patients with EGC and have raised concern about the use of endoscopic resection versus surgery in Western patients.27,31 In a review of 923 patients with T1a gastric cancer from the US Surveillance, Epidemiology, and End Results Program (SEER) database, it was observed that the overall rate of lymph node metastasis was 7.8%, more than double that observed in the Asian literature. One important note is that the incidence of lymph node metastasis differed significantly when stratified by race, with patients of Asian origin having a lower rate of lymph node metastasis than non-Asian patients (4.4% vs. 9.1%).27
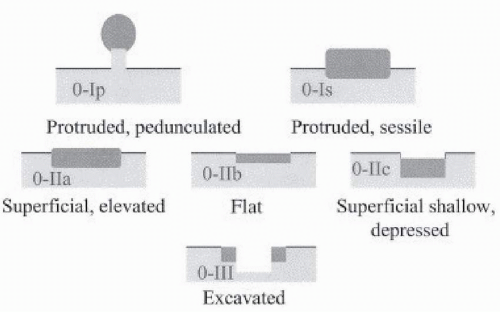
determination of suitability for endoscopic resection is based on assessment of the morphologic characteristics of the lesion on endoscopy, because endoscopic sonography and cross-sectional imaging cannot reliably exclude submucosal invasion.33
TABLE 3-2 Risk of Submucosal Invasion in Japanese Patients with Stage 0 (In Situ) Gastric Adenocarcinoma | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
and ESD (98.5% vs. 99.7%).41 Thus, on the basis of its higher en bloc resection rates and decreased local recurrence rates, ESD is the preferred technique for endoscopic resection of EGC when adequate expertise is available.32,36
TABLE 3-3 Oncologic Outcomes of Endoscopic Resection of Early Gastric Cancer | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
safety of bursectomy in an RCT in Japan among patients with T2/3 gastric cancer. In that study, 210 patients were randomly assigned to include bursectomy or not with radical gastrectomy and D2 lymphadenectomy. The morbidity (14%) and mortality (1%) rates were similar for the two groups, and complications included pancreatic fistula, abscess, and anastomotic leakage. The survival outcomes were recently published in a follow-up report.58 After a median follow-up time of 80 months, the 5-year OS (77.5% with bursectomy vs. 71.3% without bursectomy) and recurrence-free survival (RFS; 73.7% vs. 66.6%) rates were similar for the two groups. Bursectomy was associated with a 10% improved OS rate for the subgroup of patients with middleand distal-third cancers and with 20% survival in patients with serosal-penetrating tumors (T3/T4). Based on this subset analysis, the authors of this study suggest that there might be some utility of bursectomy in T3/T4 middle to distal-third cancers. However, the trial was not powered for this outcome measure. Thus, although some surgeons, predominantly in Asia, routinely perform and advocate for bursectomy, it cannot be recommended based on the available data and should not be considered a necessary component of gastrectomy for adenocarcinoma. A larger trial that includes a quality-control aspect for the bursectomy procedure is currently underway.
TABLE 3-4 Comparison of Outcomes after Total Versus Partial Gastrectomy for Cancers in the Distal Stomach | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
blood loss, decreased postoperative pain, decreased incidence of postoperative complications, and a higher likelihood of receiving adjuvant therapy.47,63,64,65 It is important that some suggest MIG has oncologic outcomes similar to those associated with open surgery.66 When considering MIG, surgeons and patients need to consider several factors, including tumor clinical stage, patient comorbidities, and surgeon experience and volume. Another important feature is that the term MIG includes myriad procedures, including distal and total gastrectomy for early and advanced gastric cancer and robot-assisted procedures.
include mainly Eastern populations with early tumors localized in the distal stomach that were detected as a result of screening protocols. These trials suggest that laparoscopic-assisted distal gastrectomy (LADG) is associated with decreased intraoperative blood loss, decreased pain scores, decreased length of stay, and improved quality-of-life measurements with longer operative times. Only one trial has reported a study on a population not located in the East.82 Main findings of this study suggested that LADG was associated with reduced estimated blood loss, shorter time to resumption of oral intake, and earlier discharge. Frequent criticisms of these RCTs have been the relatively small number of patients included and the limitation of being conducted at single centers. The Korean Laparoendoscopy Gastrointestinal Surgery Study (KLASS) Group is a multicenter effort to evaluate the oncologic feasibility of LADG against open distal gastrectomy (ODG) for EGC.87 Early safety results have been published,87 showing a lower overall complication rate for LADG (LADG vs. ODG, 13% vs. 19.9%; P = 0.001). Major intra-abdominal complication and mortality rates were similar between the two groups. Large database retrospective studies have shown a slight decrease in length of stay but no differences in terms of mortality and morbidity.69 Operative complication rates have been reported to be lower in the LADG groups in retrospective series (25.3% vs. 40.1%, P ≤ 0.001)69 and an analysis of large databases (17.5 vs. 24.4%, P ≤ 0.001),69 as well as in a prospective study.87
TABLE 3-5 Randomized Controlled Trials of Minimally Invasive Gastrectomy for Adenocarcinoma: Study Characteristics and Selection Criteria | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 3-6 Randomized Controlled Trials of Minimally Invasive Gastrectomy for Adenocarcinoma: Comparison of Operative Characteristics and Length of Stay | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TABLE 3-7 Randomized Controlled Trials of Minimally Invasive Gastrectomy for Adenocarcinoma: Morbidity and Mortality | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Kim et al87 reported short-term outcomes of a large RCT, indicating that lymph node harvesting in LADG was slightly inferior (40.5% vs. 43.7%, P < 0.001) but, nonetheless, above the recommended standard. Survival has been suggested to be similar in both groups, but prospective data are lacking.88,89 These data should become available in the next few years.87 In addition, the KLASS-03 trial (NCT01584336) is evaluating laparoscopic-assisted total gastrectomy for clinical stage I gastric cancer.
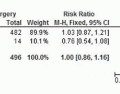
node retrieval,97,98,99,104,105,106 and, what is more important, have suggested no difference in OS.99,101,102,105,107,108,109 One RCT has reported open and laparoscopic-assisted gastrectomy (LAG) for advanced gastric cancer. In this trial 123 patients were randomly assigned, 61 to LAG and 62 to open gastrectomy. Operating time was longer in the LAG group than in the open group (267 vs. 182 minutes, P ≤ 0.001). The morbidity rate was 12% for the LAG group but 19% for the open group (P = 0.357).110 In a meta-analysis for advanced gastric cancer, Martinez-Ramos et al111 found that in comparison with an open-procedure LAG was associated with longer procedure times, decreased intraoperative blood loss, decreased length of stay, and harvesting of a similar number of lymph nodes. Choi et al112 in another meta-analysis concluded that there was no evidence to suggest that LAG is inferior to open gastrectomy for advanced gastric cancer in regard to overall and disease-free survival.
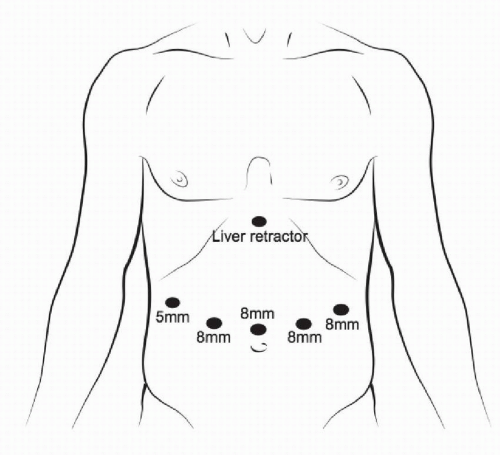 FIGURE 3-6 Port positioning and sizing specifically for robot-assisted minimally invasive gastrectomy. |
with improved short-term perioperative outcomes. Several RCTs, as well as multiple retrospective database and case-controlled series, have demonstrated that MIG is a feasible, safe technique. Emerging technologies such as robot-assisted gastrectomy have produced promising initial results and will likely play an increasing role in gastric cancer surgery.
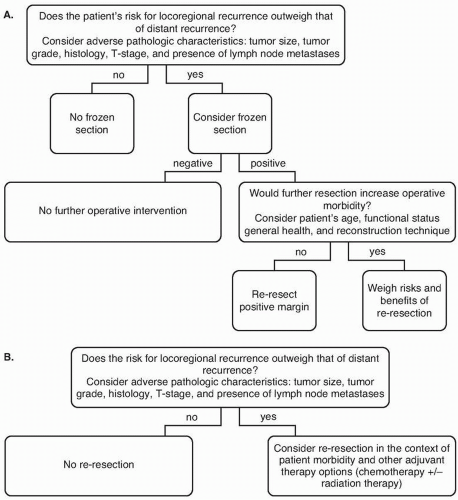
For resections of gastric adenocarcinoma, a microscopically negative (R0) resection margin should be sought.
For patients with early stage cancers of the distal stomach, the primary tumor should be resected with intent to achieve a final proximal margin of 3cm or more. For patients with later-stage distal cancers, and for patients with proximal tumors, data do not support extension of the margin to obtain any specific margin length.
Achieving greater proximal margin length must be balanced with the potential of increased morbidity with an extended resection, especially when other poor prognostic factors, such as nodal metastases, are present.
Frozen-section analysis is an appropriate method of margin evaluation intraoperatively, but gastric re-resection in the setting of a positive frozen-section margin is controversial.
question of whether there are improved outcomes associated with any resection margin result other than a microscopically negative (R0) margin.
when determining the gross in vivo margin and intraoperative decision of where to transect the stomach.
it is the effect of this conversion on recurrence and survival that must be understood to determine appropriate patient selection for this practice.
was found to be similar to that of the proximal margin, because it was associated with the presence of lymphovascular invasion, larger tumor size, and one or more positive lymph nodes.150 Although some have reported that an R1 duodenal margin was related to shortened survival, others have found it not to be associated with LRR, other recurrence, or OS.131,150,151 Re-excision of the distal margin in gastric surgery typically requires a pancreaticoduodenectomy. This aggressive management has been reported in the literature; however, these studies’ small sample sizes preclude determination of the benefit of this practice in the context of increased procedural morbidity.152,153,154 Thus, again, the clinical context must be considered, and aggressive additional surgical intervention should not be pursued in the context of aggressive, advanced disease.152
an LRR less clinically relevant in this population.151,168 Conversely, in early stage disease, an isolated LRR likely dictates patient prognosis. What is important, however, is that, as previously discussed, the event of a positive margin is associated with other poor prognostic factors, and R1 margins are less common in early stage disease.162 Additionally, in early stage disease, the event of a LRR is rare.132 Therefore, the role of re-resection or other additional therapy in the context of a positive margin must be carefully considered.
increased morbidity and perioperative mortality with this approach, but little oncologic benefit. As highlighted in Key Question 1, the debate over the optimal extent of lymphadenectomy therefore continues.
TABLE 3-8 Anatomic Definitions of Lymph Node Stations | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree