Population to be screened
Age to commence screening
Women at average risk
Annual screening mammograms
40 years
Women at an elevated risk
(a) Women with certain BRCA 1 or BRCA mutations or those who have not been tested but have first degree relatives[Mother, sisters, daughters] with such proven mutations
Yearly starting by 30 years of age but not before 25
(b) Women ≥ 20 % lifetime risk of breast cancer based on maternal or paternal family history
Yearly starting by 30 years of age but not before age 25 or 10 years before diagnosis of youngest affected relative whichever occurs later
(c) Women with mothers or sisters with premenopausal cancer
Yearly starting by 30 years of age but not before 25 or 10 years before diagnosis of youngest affected relative whichever occurs later
(d) Women with history of mantle radiation usually for Hodgkin’s lymphoma received between 10 and 30 years
Yearly starting 8 years after therapy but not before age 25
(e) Women with biopsy proven lobular carcinoma in situ, atypical lobular hyperplasia, atypical ductal hyperplasia, ductal carcinoma in situ, invasive carcinoma, ovarian carcinoma
Yearly from the time of diagnosis regardless of age
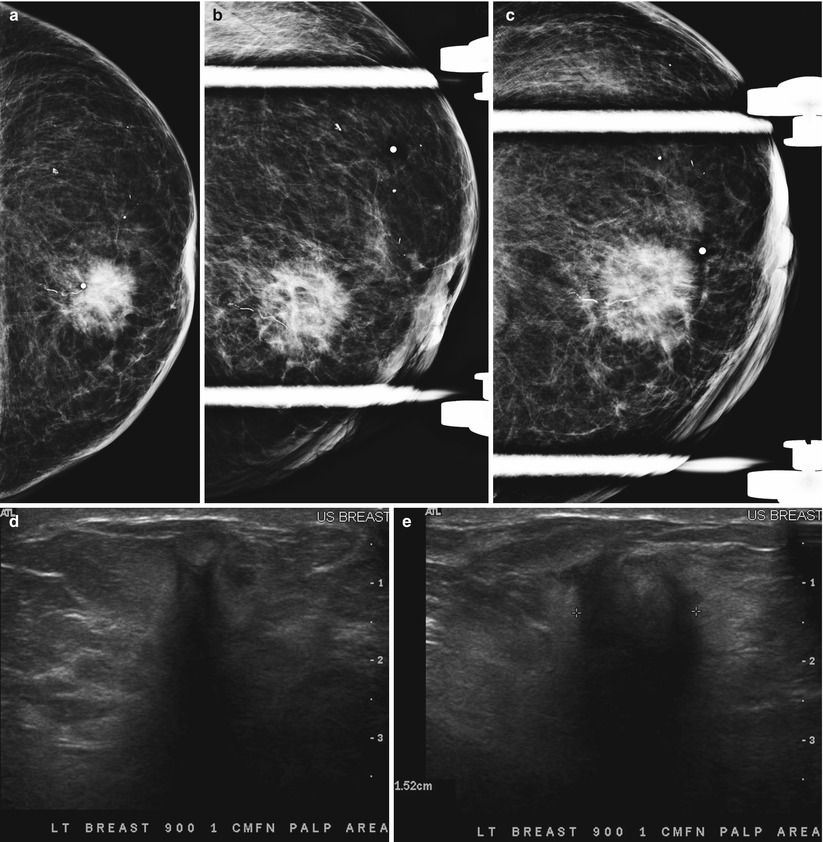
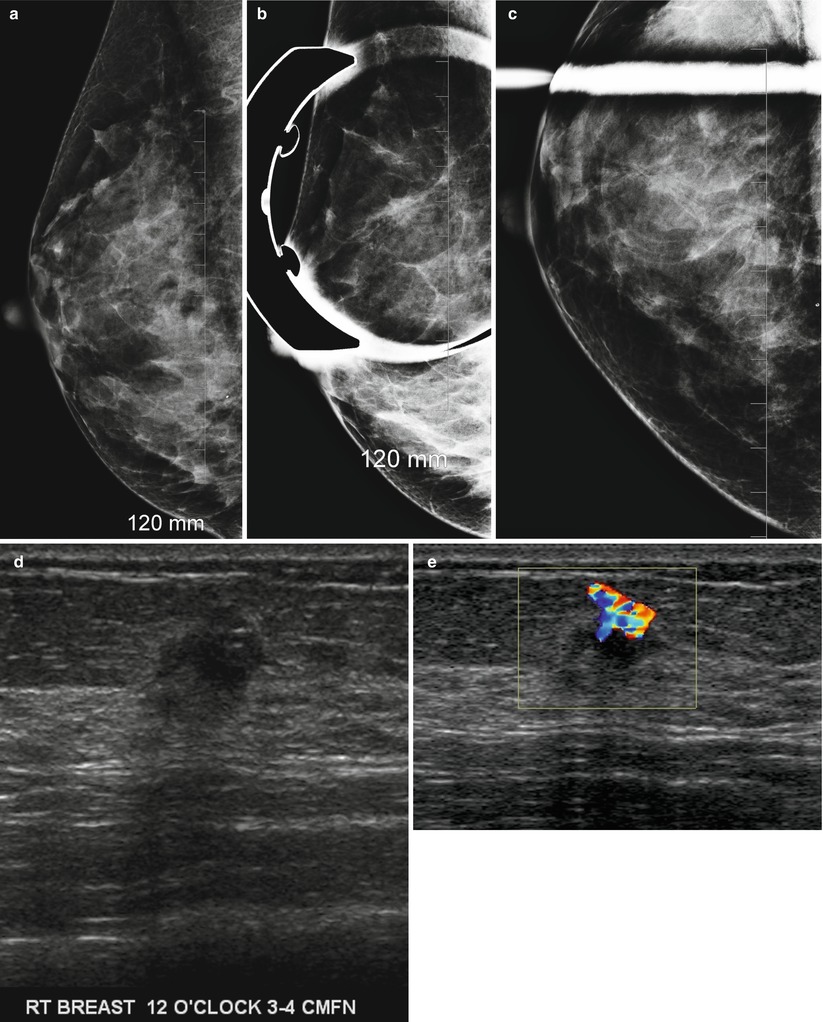
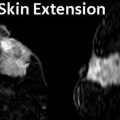
Fig. 2.1
(a–e) A 95-year-old with a palpable lump in left breast. (a) Left mediolateral view shows a 1.5 cm irregular mass. (b, c) Spot compression views show a spiculated mass. (d, e) Radial and antiradial ultrasound images demonstrate a 1.5 cm mass with malignant features. Histology showed an invasive ductal carcinoma
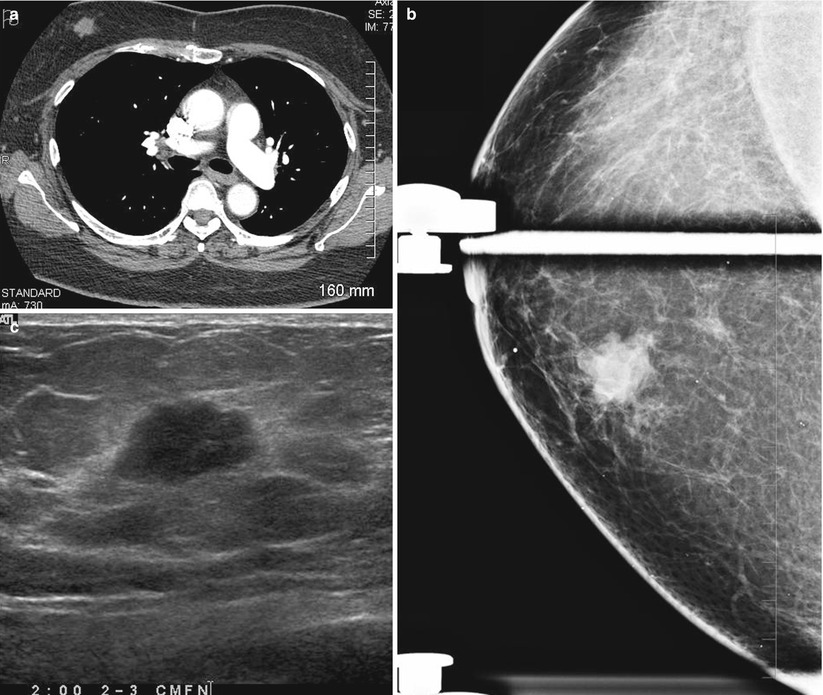
Fig. 2.2
(a–c) A 60-year-old who had not undergone screening mammogram in 4 years. (a) CT pulmonary angiogram performed for chest pain reveals a 2 cm mass in right breast. (b) Spot compression craniocaudad view of the right breast shows an irregular mass in the inner right breast. (c) Sonography reveals a 2 cm mass with irregular borders suggestive of malignancy. Histology showed an invasive ductal carcinoma
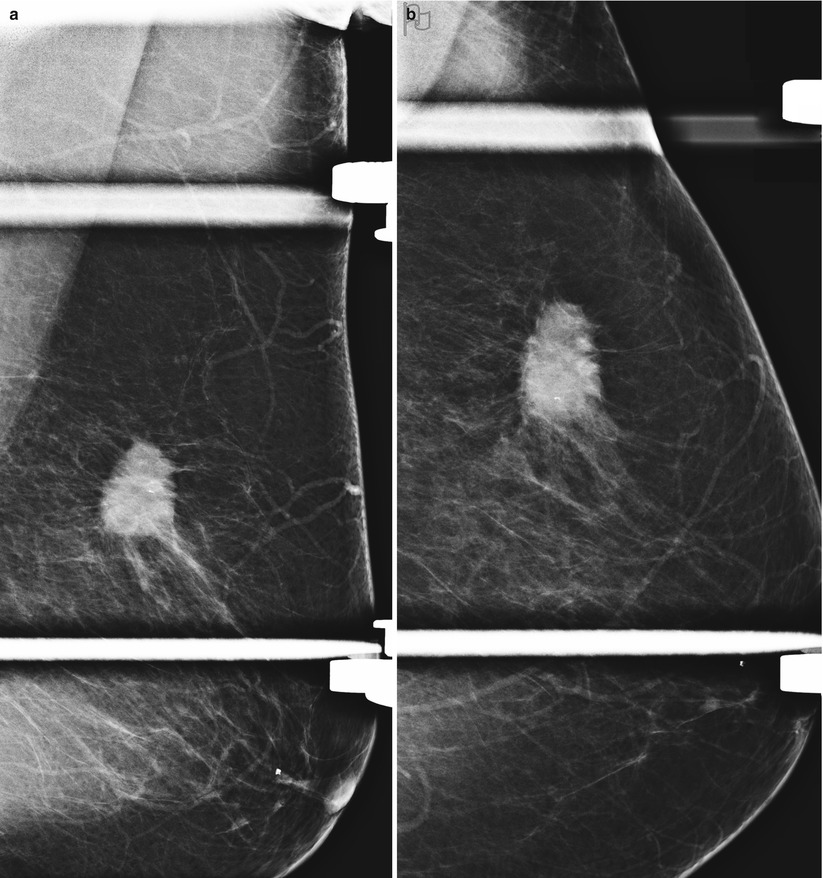
Fig. 2.3
(a–d) A 72-year-old woman not screened in 6 years. (a) Left breast mediolateral oblique view with spot compression demonstrates an irregular spiculated mass. (b) Left breast craniocaudad view with spot compression. (c, d) Left breast ultrasound shows a 3 cm mass with malignant features. Histology showed an invasive ductal carcinoma
Limitations and Potential Harm from Screening Mammography
There are some who question the benefit of screening mammography. Controversies regarding the false positives resulting from mammography, the benefit of performing screening in women in their 40s, and whether mammography overdiagnoses cancer, leading to unneeded treatment interventions, are some of the issues. Approximately 95 % of women with abnormalities on the screening mammogram do not have breast cancer [9]. In a review commissioned by the US Preventive Services Task Force, the sensitivity of mammography for a 1-year screening interval was found to be 71–96 % and substantially lower for women in their 40s. The specificity was 94–97 %; it has to be borne in mind that false positive meant recall of the patient for additional views and resolution of the abnormality in most instances without the need for a biopsy or surgical intervention. The positive predictive value of one-time mammography ranged from 2 to 12 % for abnormal results requiring further evaluation and from 12 to 78 % for abnormal results requiring biopsy. There is continued increase in predictive value with age [10].
Screening Women in Their 40s
The mammographic sensitivity is lower in women in their 40s mostly due to increased prevalence of dense breast tissue in this age group. The incidence of cancer in this age group is lower about 140 per 100,000 compared to 500 per 100,000 in women older than 50 years. An evidence-based analysis from Canada concluded that there is Level 1 evidence that screening mammography in women aged 40–49 years at average risk for breast cancer is not effective in reducing mortality [11]. The Canadian Task Force of Preventive Services supports neither the inclusion nor the exclusion of screening mammography for women in their 40s. In the USA there is disagreement among nation organizations regarding the benefit of screening in their 40s. The National Institutes of Health, the American Association for Cancer Research, and the American Academy of Family Physicians do not recommend screening women in their 40s, whereas the National Cancer Institute, the American Cancer Institute, the American Cancer Society, the American College of Radiology, and the American College of Obstetricians and Gynecologists do.
Although women in their 40s have denser breast and a lower incidence of breast cancer accounting for decreased sensitivity of mammography, in this age group, women tend to have faster growing cancers [9]. The evidence of reduction of mortality for women between 40 and 49 years is lower yet significant. A study that looked at the data from all four Swedish trials for women in this age group reported a 23 % mortality reduction at randomization achieved from a median trial time of 7 years, a median follow-up of 12.8 years, and a screening interval of 18–24 months [12]. About 18 % of cancers both in situ and malignant are reported in women between the ages of 40 and 49 in the USA. A longitudinal cohort study of 1977 women in this age group who had primary breast cancer was undertaken over an 18-year period. A significant increase in the percentage of mammography-detected cancer was seen over time (28–58 %), and a concurrent decline in patient- and physician-detected breast cancer (73–42 %), with a consequent increase in lower stage disease detection and decrease in higher stage disease [13]. A study of 31,814 average-risk women found that the positive predictive value for further evaluation was 1–4 % for women aged 40–49 years, 4–9 % for women aged 50–59 years, 10–19 % for women aged 60–69 years, and 18–20 % for women aged 70 years or older [14].
Harms of Mammography Screening
Overdiagnosis refers to diagnosis of cancers particularly DCIS [ductal carcinoma in situ] which may have never progressed to an invasive stage and resulted in death. Such patients would have undergone surgery, chemotherapy, and/or radiotherapy with consequent harm to women [15]. The presumptive evidence for “overdiagnosis” is suggested by the fact that breast cancer diagnosis in the screened group remained persistently higher even after many years when compared to the control group of non-screened women in large randomized clinical trials. This assertion is contentious because diagnosing more breast cancer cannot be somehow construed to be a bad thing, and mortality rate reduction which has been shown beyond question should be the one and only benchmark of success of screening mammography. Despite the criticism that mammography may find DCIS that may never become invasive is a moot point since the same detractors of screening have no answer to the fact that we do not know or have a means of determining which cases of DCIS will progress on to the invasive stage and which ones do not.
On the other hand two observational studies among women who underwent the current standard technique of a two view mammography and included millions of person years of observation reported a much stronger mortality reduction than what has been shown in RCTs of 30–40 % for women in their 40s. In fact RCTs tend to underestimate the benefit from screening mammography because it includes all women in the screened group who are invited to be screened including those who do not actually end up getting a mammogram and do not exclude women in the control group who may end up getting a mammogram outside the trial. As has been previously pointed out, in several RCTs, the mammographic quality was not comparable to the current standards, and one-view mammogram only was obtained which limits the cancer detection rate [16].
Interpretive accuracy varies among radiologists, especially in mammography. A study that examined the relationship between radiologists’ confidence in their assessments and their accuracy in interpreting mammograms found that confidence in mammography assessments was associated with better accuracy, especially for low-volume readers. Asking for a second opinion when confidence in an assessment is low may increase accuracy [17]. The other significant potential harm resulting from screening mammography is from false-positive results that lead to unnecessary patient anxiety and unneeded breast biopsies. Although this is a shortcoming of mammography, it is a given that any screening modality is bound to have some false positive as no test is perfect. However, much can be done to minimize the false positives, and the following section addresses ways of achieving this objective.
The most recent review of the benefits and potential harms of breast cancer screening was performed by an independent panel in the UK [18].The review was performed based on the UK screening program which offers screening for women 50 years or older once every 3 years. The review included assessment of the relative mortality benefit in women who were invited to be screened and looked at 11 randomized clinical trials with a 13-year follow-up, a mortality rate reduction of 20 % was noted, and the benefit is higher among women who underwent screening as opposed to those who were invited to and did not undergo screening. This increased benefit is difficult to ascertain. This panel looked only at RCTs that were conducted 20–30 years ago, and since then there has been significant improvement in both the quality of mammographic technique and interpretive accuracy. More recently published studies have been observational studies, namely, ecological studies, case control studies, and incidence-based mortality studies that showed a greater benefit but were not included in the review. The absolute mortality benefit is variable but was estimated to be one breast cancer death prevented for 180 women screened [18].
The overdiagnosis rate is hard to quantify and varied from 0 to 36 %. Of more importance is the fact that neither the woman nor her physician has any means of knowing which of the screen detected DCIS or invasive cancer is an “overdiagnosed case.” If it was somehow possible to distinguish at screening those cancers that would not lead to death if left untreated from those cancers that would, the overdiagnosis problem would be solved. Even DCIS that is often diagnosed on screening does not inevitably equate to overdiagnosis since 10 % of DCIS leads to subsequent development of invasive cancer even when treated with wide local excision [18]. The sources of data for overdiagnosis are few, and data are mostly based on indirect estimates. Data from three RCTs that did not screen the control group and followed them for several more years showed an estimated rate of overdiagnosis in order of 11 % from a population perspective and about 19 % from the perspective of a woman invited to screening. It has been estimated that for every 10,000 women invited to screening from 50 years onward for 20 years, there will be 681 cancers, estimated overdiagnosis rate is 129 cases, but 43 deaths from breast cancer will be prevented. An expert opinion panel after an exhaustive review of data opined that benefits of screening and benefits of better treatment are independent. Uncertainty as to whether some of the benefits in mortality rate reduction are due to better treatment is not a justification to stop screening [18].
The benefits of screening mammography have been questioned, and it has been suggested that RCTs were fundamentally flawed in design and that the results are not scientifically valid [19, 20]. An opposing view on the benefits of screening mammography that was recently published claimed that a review of clinical trials with adequate randomization did not show a statistically significant mortality rate reduction at 13 years [20]. The total rate of lumpectomies, mastectomies, and radiation therapy was increased in the screened group. When seven trials including 600,000 women were reviewed, the mortality rate reduction was seen to be only 15 % with a significant overdiagnosis and overtreatment which was estimated to be at 30 %. These authors concluded that for every 2,000 women invited for screening throughout 10 years, one breast cancer death will be prevented and ten healthy women will be treated unnecessarily. About 200 women will be subjected to anxiety and distress due to false-positive findings [20].
Nonmammographic Screening for Breast Cancer
Breast MRI and whole breast ultrasound survey have been shown to be of greater sensitivity than mammography in the early detection of breast cancers [9, 21–39]. However, unlike mammography, these two modalities have not been proved to reduce breast cancer mortality. Proof of mortality rate reduction will require a randomized controlled clinical trial involving a large number of women receiving screening with the new modality, who will then have to be followed for at least 15 years and be matched with a control group of women who receive the current standard care. The new modality being tested would have to show mortality rate reduction over and above what has been achieved with screening mammography; this is unlikely to be the case anytime in the near future [9]. At the present time, ultrasound and MRI are being used to supplement mammography for breast cancer screening in women with an elevated risk for cancer. The role of ultrasound for this reason is discussed next; the role of MRI in breast cancer screening is discussed in the chapters on breast MRI (Chaps. 8 and 9). A brief discussion on two additional examinations that have been used as supplemental tools or primary means for screening, namely, breast self-examination and clinical breast examination, follows.
Supplemental Screening with Ultrasound in Women with an Elevated Risk for Breast Cancer
In North America, breast ultrasound has been predominantly used as a targeted examination for a clinical or mammographic problem, whereas in Europe whole-breast ultrasound survey has been more prevalent [26]. It is not uncommon to identify incidental nonpalpable cancers during diagnostic sonographic evaluation of a mammographic or physical finding [26]. Mammography is known to have a limited sensitivity in women with dense breast tissue. The use of breast ultrasound as a supplemental modality for breast cancer screening has been studied in women with dense breast tissue and in those with an elevated risk for breast cancer. Dense breast tissue is by itself considered a risk factor for breast cancer [27]. It has been suggested that in women with a threefold relative risk compared with women without any known risk factors, it is enough to be categorized in the high-risk group [29]. To date, none of the major professional societies in the USA or elsewhere recommend the use of screening ultrasound for breast cancer.
A systematic search and review of studies involving mammography and ultrasound performed for screening of breast cancer found 6 cohort studies, of which only two had follow-up on patients with negative or benign findings. Screening ultrasound performed in women with American College of Radiology breast density types 2–4 identified primarily invasive cancers in 0.32 % of women. The mean tumor size was 9.9 mm, and 90 % of the cancers were node negative. Biopsy rate was high at 2.3–4.7 %, with positive predictive value of 8.4–13.7 % for those biopsied because of an abnormal finding on the ultrasound examination. The added benefit of using ultrasound to screen for breast cancers in women with a negative mammogram might be lower in women aged 50–69 years [23].
The most notable and the largest clinical trial of screening ultrasound to date is the American College of Radiology Imaging Network trial 35 (ACRIN 6666). This study was a prospective multicenter trial randomized to a group receiving ultrasound and mammographic screening and one to mammographic screening alone to compare the diagnostic yield of performance of breast ultrasound and mammography versus mammography alone in women with elevated risk of cancer [22].The criteria used in this study to determine an elevated risk for breast cancer included a personal history of breast cancer, prior atypical biopsy, and elevated risk based on the Gail or Claus model or both. A standard protocol and interpretive criteria were used. Mammography and ultrasound were performed and read independently, allowing for reducing potential biases in patient recruitment and interpretation. Data were analyzed from 2,637 patients who underwent imaging. Thirty-one cancers were detected in the study group, 11.8 per 1,000 women; the increase in the cancer detection rate because of addition of ultrasound was 4.2 per 1,000 women. The diagnostic accuracy for mammography was 0.78, for ultrasound was 0.80, and for combined mammography and ultrasound was 0.91. Ultrasound hence proved a useful supplemental modality, identifying additional small node-negative invasive cancers in this cohort of women at an elevated risk for breast cancer [22].
Breast sonography has never been studied or been advocated to be used as the only modality to screen for breast cancer. The rationale against such an approach is sound; not the least is the low yield of ultrasound alone detected breast cancers. There is, however, some data from a study in Japan that demonstrate the value of sonography when used as the only modality for screening of breast cancer in women less than 40 years of age [29]. This study was undertaken in the Ibaraki prefecture of Japan where the breast cancer screening recommendations include performing annual screening ultrasound and CBE in women of ages 30 through 56 and biannual mammography in women of ages 40 through 65. There were 12,359 women in the age group of 30–39 years who received annual screening breast ultrasound and did not undergo mammographic screening. Of these, 4,501 women also received annual CBE in addition to whole breast screening ultrasound. In young women, i.e., younger than the age of 40 years, as expected, the cancer yield was low, with a cancer detection rate of 0.04–0.07 % [34]. In those women between the ages of 40–56 years in whom both mammography and ultrasound were used, the cancer detection rate ranged from 0.13 to 0.16 % for sonography and 0.1–0.22 % for mammography. Overall, 41,653 women underwent mammography, and 48,294 women underwent CBE and breast ultrasound. The rate of detection of stage I cancers was 72 % by ultrasound, 66 % by mammography, and 42 % by CBE. Cancer detection by mammography and ultrasound was complementary. Approximately one-third of cancers would have been missed if only one of these modalities were used, which once again proves the value of supplementing ultrasound with mammography, as has been shown in the ACRIN 6666 trial [29]. There have been other studies conducted in Japan, where a significant proportion of women tend to have small breasts with dense parenchyma and are better suited for whole breast ultrasound survey. These studies have also validated use of ultrasound in the detection of small cancers in women with dense breasts [30, 31].
Breast Ultrasound: Pros and Cons (Table 2.2)
Table 2.2
Screening breast ultrasound: Pros and cons
Advantages | Disadvantages |
|---|---|
Identifies small node-negative cancers that are missed by screening mammography | Operator dependent |
Better tolerated by the patient, no ionizing radiation, no patient discomfort | Requires longer physician time compared to interpreting mammograms |
May be beneficial as a supplemental modality in women with an elevated risk for breast cancer and/or in women with a dense breast | High false-positive rate |
Biopsy of a suspicious abnormality is easier to perform than for mammographically identified abnormalities | Mortality rate reduction has not been proven in a randomized clinical trial as has been shown with mammography |
Lower sensitivity in identifying DCIS compared with mammography |
The benefits of ultrasound as a screening modality are that it does not use ionizing radiation, is well-tolerated, does not require intravenous contrast administration, and is optimally amenable for percutaneous biopsy guidance. Ultrasound is able to identify small nonpalpable masses while undeterred by presence of dense breast tissue, which is an inherent limitation of mammography. More than 90 % of cancers identified at sonography are in women with >50 % of dense breast tissue [32, 33]. In addition ultrasound is a useful supplemental tool in identifying small cancers with subtle findings on a mammogram (Fig. 2.4a–e).