Fig. 3.1
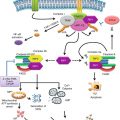
Role of innate immune receptors in the regulation of antitumor immunity. (a) The functions of the innate immune system are regulated by various receptors expressed in immune cells. C-type lectin-like receptors (CLRs) regulate recognition and uptake of antigens (such as DEC-205), the interactions between immune cells (such as the interaction between DC-SIGN on APCs and ICAM-3 on T cells), and the recognition of dead cells, such as CLEC-9A which recognizes necrotic cells and enhances cross-presentation of antigens derived from necrotic cells to CD8+ T cells. Members of B7 family regulate the functions of APCs, such as B7-H1 and B7-H4, which have immune suppressive effects, while other members regulate the interaction with immune cells, such as B7-H3, which interacts with NK cells and suppress its functions, and B7-1/B7-2 which regulates APCs-T-cell interactions. Phagocytosis receptors expressed on APCs interact with ligands on apoptotic cells and mediate its removal by APCs. In some cases, ligand-phagocytosis receptor interactions (such as CD47-SIRP-α) provide an inhibitory signal which blocks phagocytosis, a system utilized by tumors to evade immune machineries. (b) The balance between activating and inhibiting signals is critical for NK cell activities. Upon interaction with corresponsive ligands, activating and inhibitory receptors deliver a signal which is mediated by ITAM and ITIM in their cytoplasmic domain. Phosphorylated ITAM motifs in activating receptors recruit adaptor proteins which activate downstream signaling pathways, while phosphorylated ITIM motifs in inhibitor receptors recruit proteins such as SHP-1 which dephosphorylates downstream signal molecules and inhibit NK activities
Manipulation of phagocytic systems has emerged as one of the tumor immune evasion machineries, and pharmacological targeting of these pathways provides a feasible option to augment host immune responses and eradicate tumors. For example, blocking CD47 with a monoclonal antibody triggers tumor destruction by inducing phagocytosis of malignant cells [90, 92], and the treatment with anti-MFG-E8 antibodies elicits potent antitumor responses in combination with conventional anticancer drugs [93].
3.3.5 C-Type Lectin-Like Receptors (CLRs)
Carbohydrate-binding C-type lectin and lectin-like receptors (CLRs) are a large family of molecules expressed in innate immune cells and play an important role in the regulation of antitumor immunity. For example, the interaction between DC-SIGN (dendritic cell-specific ICAM-3 grabbing non-integrin) and ICAM-3 (intercellular adhesion molecule 3) facilitates the cross talk between DCs and T lymphocytes, hence influences immunogenic responses against pathogens and tumors [94]. DEC-205 is highly expressed on DCs and promotes cross-presentation of tumor antigens to cytotoxic T lymphocytes [95]. Indeed, agonistic antibody targeting DEC-205 elicits potent antitumor immunity and durable tumor regression in various murine tumor models [96]. In addition, C-type lectin domain family 9A (CLEC9A) utilizes necrotic cells for uptake, antigen presentation, and immune response, hence raising the possibility that CLEC9A-mediated recognition of immunogenic antigens may enhance antitumor immunity and clinical responses [97] (Fig. 3.1a). Therefore, CLRs serve as promising candidates for improving therapeutic responses to cancer immunotherapy. Moreover, deep understanding of the mechanism through which CLRs regulate innate immune response will lead to improvement in cancer vaccines.
3.3.6 NK Cell Receptors
NK cells possess various sets of pattern-recognition receptors which activate or suppress immune responses upon encountering their target cells. The balance between activation and inhibition signals is carefully mediated by signals triggered by both activation and inhibition receptors in combination with cytokines. Signals delivered from NK receptors mainly mediate through immunoreceptor tyrosine-based activation motif (ITAM) and immunoreceptor tyrosine-based inhibition motif (ITIM). ITAM and ITIM bear conserved sequences of four amino acids repeated twice in the cytoplasmic tails of NK cell receptors. Phosphorylation of tyrosine within ITAM motifs recruits adaptor proteins such as DNAX-activating protein-12 (DAP12) and DNAX-activating protein-10 (DAP10) involved in activating downstream signaling pathways. On the other hand, phosphorylation of tyrosine within ITIM motifs recruits proteins such as SHP which dephosphorylates downstream signal molecules to inhibit NK stimulation [98] (Fig. 3.1b).
Tumor cells evolve multiple strategies to evade NK cells by modulating ligand expression, ligand shedding, and upregulation of MHC molecules, in addition to the production of immunosuppressive cytokines. Thus, it is important to understand the underlying mechanism of NK cell activation and inhibition by their receptors, which eventually regulate immunosurveillance. NKG2D is a homodimeric C-type lectin-activating receptor expressed on NK, NKT, and activated CD8+ T cells [16, 99]. Ligands for NKG2D include stress-induced proteins, such as MHC class I chain-related A and B (MICA and MICB) as well as unique long 16 binding proteins (ULBPs) in human [99] and RAE1, H60, and Mult1 in mice. NKG2D ligands are upregulated in stress conditions, such as viral infection and transformation [99–102]. Several signaling pathways are involved in the induction of NKG2D ligands, including HSP70-mediated cellular stress [101] and ATM/ATR-mediated DNA damage pathways [103]. Importantly, blocking of NKG2D pathways increases the susceptibility of mice to chemically induced carcinogenesis [104], indicating the importance of NKG2D in tumor immunosurveillance. Natural cytotoxicity receptor (NCR) family consists of three activating receptors: NKp30, NKp44, and NKp46, which are able to induce a strong cytotoxic reaction by NK cells. Expression levels of NCRs are correlated with cytotoxic ability of NK cells. MHC class I molecules counteract with NCR-mediated activation signals; in addition, the loss of MHC-I molecules, frequently observed in transformed cells, activates NCRs on NK cells [105–107].
Killer cell immunoglobulin-like receptors (KIRs) are a family of cell surface molecules expressed on NK cells. KIRs have many members divided into two groups depending on the number of extracellular Ig domains (2D or 3D) or the length of their cytoplasmic tail, long vs. short (L or S). L-forms are shown to have inhibitory functions, while S-forms enhance cytotoxic activities of NK cells in DAP12-mediated signal pathways. KIRs regulate NK cells’ killing function through the interaction with MHC class I molecules [100, 108].
The interaction between inhibitory KIRs and normal MHC-I molecules inhibits NK cell stimulation. Correspondingly, NK cell stimulation can occur due to an interaction between activating KIRs and polymorphic self-MHC class I molecules. Inhibitory KIRs were shown to be involved in the escape mechanism of acute myeloid leukemia (AML) from NK cell immune surveillance, mechanism of which includes a mismatch between donor KIRs and recipient human leukocyte antigen ligands [109]. Thus, the understanding of KIR-mediated recognition of missing self is important in the treatment of AML [110].
Ly49 family is a large group of receptors expressed in mice but not in humans [111]. Functionally Ly49 is similar to human KIRs, containing both activating and inhibitory receptors. Inhibitory Ly94 receptors possess ITIM motifs which recruit SHP-1 to trigger an inhibitory signal, while activation receptors interact with DAP12 to activate lytic machinery in NK cells [112]. Ly49H is an activating NK receptor which recognizes m157 glycoprotein encoded by mouse cytomegalovirus (MCMV). Upon interaction with m157, Ly49H associates with DAP12 and DAP10 to stimulate NK cell-mediated cytotoxic activities against infected cells [113], suggesting a role for Ly49H in the protection against viral infection-associated tumors [114].
DNAM-1 (CD226) is an adhesion molecule expressed on the surface of NK cells, monocytes, and a subset of T cells. DNAM-1 belongs to the immunoglobulin superfamily containing 2 Ig-like domains of the V-set. DNAM-1 is reported to bind to two ligands: CD112 and CD155 [115]. CD112 and CD155 are highly expressed in some tumors like melanoma and neuroblastoma. Importantly, neuroblastoma cells that do not express CD112 and CD155 are resistant to NK cells, indicating that NK lysis of this neuroblastoma cells requires DNAM-1 interaction with its ligands on tumor cells [116].
3.3.7 B7 Family
B7 family consists of co-stimulatory and co-inhibitory receptors found on activated APC and T cells, which regulate the interaction between APCs and T cells. B7-1 and B7-2 are expressed on APCs and are involved in the stimulation of T-cell response. B7-1 and B7-2 on APCs serve as co-stimulatory molecules and play a critical role in regulating antitumor immune responses through reciprocal interaction of their receptor CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) on T lymphocytes [117, 118]. B7-H1 (PD-L1) expression in DCs is induced by IL-10 and VEGF at ovarian tumors [119]. B7-H1 on DCs suppresses IL-12 and promotes IL-10 secretion, creating an immunosuppressive tumor environment. Moreover, the blockade of B7-H1 enhances antitumor immunity by DC-mediated T-cell activation [119, 120]. In addition, treatment with PD-1 neutralizing antibodies has been found to decrease tumor growth and metastasis in B16 melanoma and colon cancer models [121, 122]. B7-H3 on APCs bind to an unidentified receptor on NK cells and transduce an inhibitory signal which suppress cytotoxic activities of NK cell. In addition, blocking of B7-H3 could restore the antitumor effects of NK cells [116]. Finally, B7-H4 promotes protumorigenic and immunosuppressive phenotypes of macrophages; for example, the blockade of B7-H4 normalized immunogenicity of macrophages and augmented antitumor immunity in ovarian tumor tissues [123] (Fig. 3.1a).
3.4 The Role of Effectors Produced from Innate Immune Cells in Cancer and Antitumor Immunity
3.4.1 Interferons (IFNs)
Type I IFNs are produced by many different cells in response to viral or bacterial infections. Type I IFNs (IFN-α/IFN-β) enhance proliferation and activation of innate immune cells such as DCs, macrophages, and NK cells [124]. In addition, they stimulate antigen processing and presentation to antigen-specific lymphocytes, which greatly contribute to tumor immunosurveillance [125]. The importance of type I IFNs in tumor immunosurveillance also validated enhanced susceptibility to tumorigenesis by treatment with anti-IFN-α/IFN-β neutralizing antibodies or in mice with targeted mutations of type I IFN receptor [126, 127].
Type II IFN (IFN-γ) is a cytokine involved in the activation of adaptive immune cells. IFN-γ is primarily produced by various innate immune lymphocytes such as NK, NKT, and γδ-T cells and plays a critical role in the induction of Th1 immune responses and the production of NO and ROS by macrophages, leading to enhanced cytotoxic activities against transformed cells [128]. IFN-γ has an important role in the protection against transplanted tumors or chemically induced tumors by increasing intrinsic immunogenicity of tumor cells [129, 130]. IFNGR−/− mice or mice deficient in IFN-γ-downstream signaling molecule Stat-1 developed tumors more rapidly and in greater frequencies compared to wild-type mice [131, 132]. Thus, IFN-γ-mediated regulation of tumor immunogenicity has a great impact on innate immunity and tumor immunosurveillance.
3.4.2 Other Cytokines
Interleukins have an important role in regulating innate immune functions in tumor microenvironments. Several cytokines, such as IL-2, IL-12, IL-18, IL-15, and IL-21, serve as NK cell-stimulants, competent in targeting transformed cells. Mice deficient for IL-12p40 are susceptible to carcinogen-induced tumorigenesis; in addition, IL-21−/− mice showed reduced colitis-associated cancers [133], indicating the role of these cytokine in protecting hosts from arising tumors. With respect to the mechanisms of action, NKG2D systems are involved in the enhancement of NK cell cytotoxic activities by all cytokines suggested above, and perforin-granzyme pathways play an important role in exerting NK cell cytolysis by IL-18. Moreover, IL-21 induces NK cell effector functions by increasing sensitivities to IFN-γ, and IL-15 regulates survival, activation, and proliferation of NK cells [134]. Cytokines produced from innate immune cells serve as feasible adjuvants in activating antitumor responses in patients with advanced cancer. For example, the systemic administration of high doses of recombinant IL-2 or the adaptive transfer of IL-2-stimulated NK cell can trigger potent antitumor responses and mediate durable tumor regressions in patients with advanced melanoma and renal cell carcinoma [135]. The clinical efficacy of IL-12 has been evaluated as a monotherapy or in combination with other immunotherapies in patients with cancer; however, they did not induce durable clinical responses [136, 137].
Several cytokines antagonize immunogenic potential of tumors and innate lymphocytes. IL-10 downregulates the expression of immunogenic cytokines, such as IFN-γ, IL-2, TNF-α, and GM-CSF, and also suppresses antigen presentation by APCs. On the other hand, the carcinogen-mediated tumor incidence was increased in IL-10-knockout mice, whereas IL-10 overexpression protects mice from arising tumors [138]. Thus, IL-10 has a complex role in tumorigenesis, and the pro- and antitumor effects of IL-10 may depend on the different experimental models. TGF-β is a regulatory cytokine which has important roles in the regulation of immune responses and immune tolerance as well as carcinogenesis [139, 140]. TGF-β can inhibit the activities of NK cells through the suppression of IFN-γ production [141], as well as the downregulation of activating receptors such as NKp30 and NKG2D [142]. On the other hand, TGF-β negatively regulates recruitment and differentiation of myeloid-derived suppressor cells (MDSCs) in tumor tissues derived from mammary carcinomas, contributing to enhanced host immunity and tumor rejection [143]. Therefore, TGF-β has different roles in antitumor immunity and tumorigenicity, which are in part dependent on the phase of tumor progression and different cellular components in tumor microenvironments [144]. Vascular endothelial growth factor-A (VEGF-A) also plays a critical role in suppressing DC maturation and differentiation, therefore impacting tumor immunogenicity and host immunosurveillance [145]. Thus, various cytokines are responsible for attenuating immunogenic potentials of innate immune systems in tumors.
Several cytokines derived from innate lymphocytes contribute to smoldering inflammation and tumor progression. IL-23-IL-17 pathway operated in endogenous tumor microenvironments represents prototypical mediators which promote tumor-associate inflammation. IL-23 promotes tumor cell growth and invasion through upregulation of proteins of the matrix metalloproteinase-9 (MMP9), COX-2, and angiogenesis. In contrast, IL-23−/− mice showed reduced inflammation and thus attenuated tumor formation [146]. IL-17 is elevated in various tumors, where it plays an important role in tumor growth. IL-17 can enhance tumor growth by direct effects on tumor cells and tumor-associated stromal cells by activating IL-6-Stat3 pathways [147]. Furthermore, the altered composition of commensal microbes and disruption of epithelial barrier functions facilitate differentiation of IL-17-producing T lymphocytes by IL-23 from myeloid cells in intestine, leading to increased colon tumorigenesis [148, 149].
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is produced in vivo by many cells including mast cells, macrophages, T cells, fibroblasts, and endothelial cells in response to immune activation and proinflammatory cytokines. GM-CSF creates an immunosuppressive tumor microenvironment by differentiating immature myeloid-derived suppressor cells (MDSCs) into tumor tissues [150]. On the other hand, the therapeutic administration of GM-CSF has been emerged as a potent immunogenic adjuvant to stimulate antitumor immunity by enhancing APC functions [151].
Macrophage colony-stimulating factor M-CSF (also known as CSF-1) is a dimeric polypeptide growth factor which regulates the proliferation, differentiation, and survival of macrophages and their bone marrow progenitors. CSF-1 expression is elevated in different tumors and is found to be accompanied by high grade and poor prognosis [152]. Targeting of CSF-1 has been evaluated in preclinical and clinical studies [153]. The administration of anti-CSF1R-neutralizing antibody (AFS98) or a CSF-1R inhibitor (Ki20227) resulted in reduced numbers of tumor-infiltrated macrophages in an implanted osteosarcoma model and reduced vascularization, angiogenesis, and tumor growth [154, 155].
3.4.3 Chemokines
Chemokines are small cytokines secreted by many cell types in response to pathological conditions, in order to activate and attract effector cells which express appropriate chemokine receptors. Two types of chemokines have been identified: CC chemokines that are chemotactic for monocytes and CXC chemokines which attract polymorphonuclear leukocytes (PMNs). Chemokines have a central role in tumor progression through the recruitment of innate immune cells into tumor site. Most studies have focused on CCL2 and CCL5 as the major chemokines in tumor microenvironment.
CCL2 (MCP-1) is produced by tumor cells and tumor-associated stromal cells and attracts CCR2+ inflammatory monocytes to the tumor microenvironment, which differentiate into tumor-associated macrophages and promote tumor aggressiveness, and the blockade of CCL2-CCR2 signaling by neutralizing antibodies suppresses metastasis and prolongs overall survival of tumor-bearing mice [156]. The levels of CCL2 expression and macrophage infiltration into tumors are correlated with poor prognosis and metastases in human breast cancer, suggesting significance of CCL2-mediated immune regulation in cancer patients [157].
CCL5, another important chemokine, plays an important role in the recruitment of monocytes into the tumor microenvironment [158]. CCL5 induces expression of CCL2, CCL3 (MIP-α), CCL4 (MIP-β), and CXCL8 (IL-8) by monocytes, which leads to the recruitment of myeloid cells into tumor site [159]. CCL5 also induces CCR1 expression on monocytes [160]. Hence, chemokines lead to the recruitment of monocytes, which produce more chemokines to further attract more monocytes as well as other leukocytes into the tumor site. CCL5 enhances antitumor immune responses against tumors [161], while it promotes tumorigenesis and metastases in some conditions [162, 163]. These findings suggest dual function of CCL5 in cancer and antitumor immunity.
Taken together, the dynamic interactions between tumor cells and innate immune cells governed by chemokine networks play a pivotal role in the regulation of tumor immunosurveillance and tumorigenicity (Fig. 3.2).
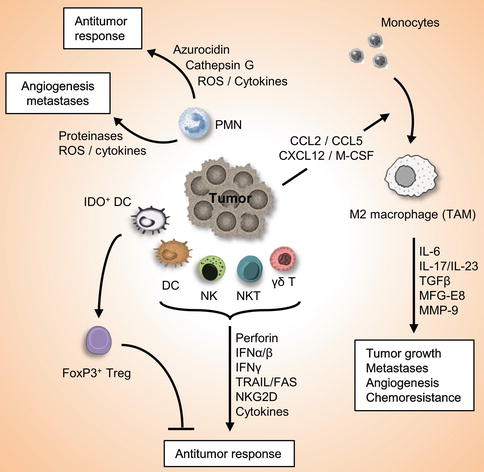

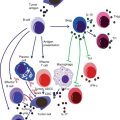
Fig. 3.2
Role of the innate immune system in cancer and antitumor immunity. Innate immune system serves as the first defense line against cancers. Innate immune cells such as DC, NK, NKT, γδ-T cells are attracted into the tumor site, where they recognize the transformed cells and release multiple factors which initiate an antitumor immune response. On the other hand, other innate immune cells may also involve in the promotion of tumor growth, angiogenesis, and metastasis. For example, IDO+ DC induces differentiation of FoxP3+Treg cells which suppress antitumor immunity, and molecules released by PMNs may have protumorigenic or antitumor effects. Furthermore, tumors secrete chemokines and cytokines that attract inflammatory monocytes into the tumor microenvironment and induce its differentiation into M2 macrophages, which play important roles in tumor progression, metastases, angiogenesis, and chemoresistance
3.5 Concluding Remarks
Innate immune system serves as the first line of defense against pathogens and cancers. In tumors, innate immune cells are attracted into the tumor site. Factors released from stressed cells at the tumor microenvironment, such as PAMPs and DAMPs, are recognized by another set of receptors, including TLRs, RLRs, and NLRs, which trigger distinct innate signaling pathways; these pathways lead to maturation, activation, as well as production of cytokines and chemokines from immune cells, to attract more immune cells into the tumor site and initiate an immune response against tumor cells. Thus, a deep knowledge of the role of innate immune system in tumor immunity and tumorigenesis is critical to develop new strategies for the immunotherapy of cancer.
Acknowledgments
We apologize to the authors whose work could not be cited due to space constraints.
This study is partially supported by a Grant-in-Aid for Scientific Research and Scientific Research for Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and the Ministry of Health, Labour and Welfare, The Naito Foundation, and the Astellas Foundation for Research on Metabolic Disorders (M.J.).
References
1.
Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6.PubMed
2.
Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71.PubMed
3.
Seliger B, Maeurer MJ, Ferrone S. Antigen-processing machinery breakdown and tumor growth. Immunol Today. 2000;21:455–64.PubMed
4.
Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. 2012;13:832–42.PubMedCentralPubMed
5.
Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7.PubMed
6.
Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3:999–1005.PubMedCentralPubMed
7.
Zou W. Regulatory T, cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307.PubMed
8.
Smyth MJ, Cretney E, Takeda K, Wiltrout RH, Sedger LM, Kayagaki N, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) contributes to interferon gamma-dependent natural killer cell protection from tumor metastasis. J Exp Med. 2001;193:661–70.PubMedCentralPubMed
9.
Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002;71:173–83.PubMed
10.
Bottino C, Moretta L, Pende D, Vitale M, Moretta A. Learning how to discriminate between friends and enemies, a lesson from natural killer cells. Mol Immunol. 2004;41:569–75.PubMed
11.
Sinkovics JG, Horvath JC. Human natural killer cells: a comprehensive review. Int J Oncol. 2005;27:5–47.PubMed
12.
Johnsen AC, Haux J, Steinkjer B, Nonstad U, Egeberg K, Sundan A, et al. Regulation of APO-2 ligand/trail expression in NK cells-involvement in NK cell-mediated cytotoxicity. Cytokine. 1999;11:664–72.PubMed
13.
Mirandola P, Ponti C, Gobbi G, Sponzilli I, Vaccarezza M, Cocco L, et al. Activated human NK and CD8+ T cells express both TNF-related apoptosis-inducing ligand (TRAIL) and TRAIL receptors but are resistant to TRAIL-mediated cytotoxicity. Blood. 2004;104:2418–24.PubMed
14.
Gołab J. Interleukin 18-interferon γ inducing factor-a novel player in tumour immunotherapy? Cytokine. 2000;12:332–8.PubMed
15.
Zamai L, Ponti C, Mirandola P, Gobbi G, Papa S, Galeotti L, et al. NK cells and cancer. J Immunol. 2007;178:4011–6.PubMed
16.
Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–9.PubMed
17.
Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–13.PubMed
18.
Elliott JM, Wahle JA, Yokoyama WM. MHC class I-deficient natural killer cells acquire a licensed phenotype after transfer into an MHC class I-sufficient environment. J Exp Med. 2010;207:2073–9.PubMedCentralPubMed
19.
Smyth MJ, Thia KY, Cretney E, Kelly JM, Snook MB, Forbes CA, et al. Perforin is a major contributor to NK cell control of tumor metastasis. J Immunol. 1999;162:6658–62.PubMed
20.
Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–41.PubMedCentralPubMed
21.
Jinushi M, Takehara T, Tatsumi T, Hiramatsu N, Sakamori R, Yamaguchi S, et al. Impairment of natural killer cell and dendritic cell functions by the soluble form of MHC class I-related chain A in advanced human hepatocellular carcinomas. J Hepatol. 2005;43:1013–20.PubMed
22.
Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–33.PubMedCentralPubMed
23.
Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “l’union fait la force”. Blood. 2005;106:2252–8.PubMed
24.
Akazawa T, Ebihara T, Okuno M, Okuda Y, Shingai M, Tsujimura K, et al. Antitumor NK activation induced by the Toll-like receptor 3-TICAM-1 (TRIF) pathway in myeloid dendritic cells. Proc Natl Acad Sci U S A. 2007;104:252–7.PubMedCentralPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree