Although melanoma is generally considered a relative radioresistant tumor, radiation therapy (RT) remains a valid and effective treatment option in definitive, adjuvant, and palliative settings. Definitive RT is generally only used in inoperable patients. Despite a high-quality clinical trial showing adjuvant RT following lymphadenectomy in node-positive melanoma patients prevents local and regional recurrence, the role of adjuvant RT in the treatment of melanoma remains controversial and is underused. RT is highly effective in providing symptom palliation for metastatic melanoma. RT combined with new systemic options, such as immunotherapy, holds promise and is being actively evaluated.
Key points
- •
Definitive radiation plays an important role in early stage ocular melanoma with high local control and organ preservation.
- •
Definitive radiation therapy (RT) may be a viable option for lentigo maligna, lentigo maligna melanoma, and unresectable mocusal melanoma.
- •
Adjuvant RT following lymphadenectomy in node-positive melanoma prevents local and regional recurrence; however, it does not improve survival.
- •
Palliative radiation treatment is an important treatment option for metastatic melanoma, particularly with new stereotactic radiosurgery and stereotactic body radiotherapy techniques.
- •
A combination of radiation treatment and immunotherapy holds promise and is being actively evaluated.
Introduction
Radiation therapy (RT) works by damaging the DNA of cancer cells.
Radiation treatment techniques are divided into the following catagories.
External Beam Radiotherapy or Teletherapy
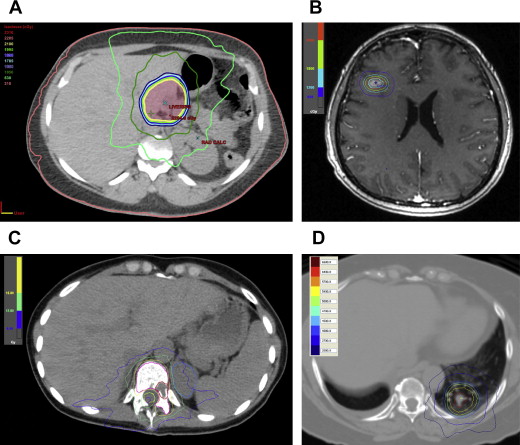
RT is delivered from a relatively distant source. The most common equipment is linear accelerators (LINAC). LINAC can produce both electron beams, suitable for superficial targets, and radiographic beams, suitable for deeper internal targets. The external beam radiotherapy (EBRT) radiation therapy treatment has been revolutionized with the advancement of computer software technology, high-resolution computed tomography (CT) and MRI imaging, and advanced delivery techniques. It has evolved from 2-dimensional RT to 3-dimensional conformal RT, and currently, intensity-modulated RT, volumetric-modulated arc therapy as well as special techniques, such as stereotactic radiosurgery (SRS) and stereotactic body radiotherapy (SBRT) ( Fig. 1 ). Besides radiography (photon), proton radiation treatment is a special form of EBRT using charged particles. Protons penetrate tissue to a certain depth and deposit the energy in the tissue in a sharp peak, known as the Bragg peak. The Bragg peak of physical dose distribution permits the accurate concentration of the dose on the tumor, thus sparing the adjacent normal tissues. It is particularly appealing for pediatric populations, and cancers close to critical structures, such as skull base, spine, and uveal melanoma. Other particle radiation also has been used for clinical treatment, including neutron, and carbon ions. However, their availability is very limited.
Brachytherapy
The radiation source is placed inside or next to the treatment area. Brachytherapy (BT) has the advantage of delivering high doses of radiation to the tumor while reducing the dose to the surrounding normal tissues. However, its use is greatly limited by the location and accessibility of the tumors. Invasive procedures are needed for access to internal organ or deep target.
Unsealed Source Radiotherapy
The soluble forms of radioactive substances are administered to the body by injection or ingestion. Examples are 131 iodine, 90 yttrium (90 Y) resin microsphere, and 223 radium dichloride.
Historically, melanoma is considered a relative radioresistant tumor. This notion primarily arose from cell culture studies, which showed a broad shoulder in the cell survival curves, suggesting a high repair capacity. The high ability to repair DNA damage would make melanoma cells more sensitive to large RT dose per fraction. Early clinical observations with large dose per fraction treatment resulted in conflicting results. A multicenter randomized phase III trial (RTOG 83-05) attempted to address this question. In this trial, 137 patients with measurable lesions were randomized to 2.5 Gy for 20 fractions and 8 Gy for 4 fractions. No difference in local control was observed. Unfortunately, the duration of response or survival was not reported. Retrospective studies comparing conventional and hypofractionated regimens in the adjuvant setting also failed to find significant differences. On the other hand, a prospective randomized trial of different hypofractionated regimens (9 Gy × 3 vs 8 Gy × 5, 2 fractions per week) resulted in similar durable complete responses. Taken together, the bias is to treat with the hypofractionated schedule with a fraction dose of 2.5 Gy or higher in the absence of contraindications. It is more convenient and generally well tolerated with a low risk of late complications.
Considering the treatment intent and relation with other modalities, the role of RT for melanoma can be divided into definitive treatment, adjuvant treatment, and palliative treatment.
Introduction
Radiation therapy (RT) works by damaging the DNA of cancer cells.
Radiation treatment techniques are divided into the following catagories.
External Beam Radiotherapy or Teletherapy
RT is delivered from a relatively distant source. The most common equipment is linear accelerators (LINAC). LINAC can produce both electron beams, suitable for superficial targets, and radiographic beams, suitable for deeper internal targets. The external beam radiotherapy (EBRT) radiation therapy treatment has been revolutionized with the advancement of computer software technology, high-resolution computed tomography (CT) and MRI imaging, and advanced delivery techniques. It has evolved from 2-dimensional RT to 3-dimensional conformal RT, and currently, intensity-modulated RT, volumetric-modulated arc therapy as well as special techniques, such as stereotactic radiosurgery (SRS) and stereotactic body radiotherapy (SBRT) ( Fig. 1 ). Besides radiography (photon), proton radiation treatment is a special form of EBRT using charged particles. Protons penetrate tissue to a certain depth and deposit the energy in the tissue in a sharp peak, known as the Bragg peak. The Bragg peak of physical dose distribution permits the accurate concentration of the dose on the tumor, thus sparing the adjacent normal tissues. It is particularly appealing for pediatric populations, and cancers close to critical structures, such as skull base, spine, and uveal melanoma. Other particle radiation also has been used for clinical treatment, including neutron, and carbon ions. However, their availability is very limited.
Brachytherapy
The radiation source is placed inside or next to the treatment area. Brachytherapy (BT) has the advantage of delivering high doses of radiation to the tumor while reducing the dose to the surrounding normal tissues. However, its use is greatly limited by the location and accessibility of the tumors. Invasive procedures are needed for access to internal organ or deep target.
Unsealed Source Radiotherapy
The soluble forms of radioactive substances are administered to the body by injection or ingestion. Examples are 131 iodine, 90 yttrium (90 Y) resin microsphere, and 223 radium dichloride.
Historically, melanoma is considered a relative radioresistant tumor. This notion primarily arose from cell culture studies, which showed a broad shoulder in the cell survival curves, suggesting a high repair capacity. The high ability to repair DNA damage would make melanoma cells more sensitive to large RT dose per fraction. Early clinical observations with large dose per fraction treatment resulted in conflicting results. A multicenter randomized phase III trial (RTOG 83-05) attempted to address this question. In this trial, 137 patients with measurable lesions were randomized to 2.5 Gy for 20 fractions and 8 Gy for 4 fractions. No difference in local control was observed. Unfortunately, the duration of response or survival was not reported. Retrospective studies comparing conventional and hypofractionated regimens in the adjuvant setting also failed to find significant differences. On the other hand, a prospective randomized trial of different hypofractionated regimens (9 Gy × 3 vs 8 Gy × 5, 2 fractions per week) resulted in similar durable complete responses. Taken together, the bias is to treat with the hypofractionated schedule with a fraction dose of 2.5 Gy or higher in the absence of contraindications. It is more convenient and generally well tolerated with a low risk of late complications.
Considering the treatment intent and relation with other modalities, the role of RT for melanoma can be divided into definitive treatment, adjuvant treatment, and palliative treatment.
Definitive radiation therapy for the melanoma
Adequate surgery offers the best chance of local control and cure for primary melanoma. Definitive RT may be considered in certain special situations, such as inoperability due to medical comorbidities, location, or patient refusal.
Definitive Radiation Therapy for Lentigo Maligna and Lentigo Maligna Melanoma
Lentigo maligna (LM) and lentigo maligna melanoma (LMM) frequently affect elderly patients and may involve large areas of the face near critical structures. Definitive RT has been used with good long-term local control with acceptable cosmetic and functional outcomes. A pooled analysis of 349 patients with LM treated with definitive RT showed a 5% local recurrence rate. Salvage was successful in most of the recurrent LM by further RT, surgery, or other therapies. A recent retrospective comparative study also revealed no statistical significant difference in outcome between surgery and RT for cutaneous melanoma.
Definitive Radiation Therapy for Mucosal Melanoma
In a series of 28 patients with mucosal melanoma, actuarial local control of 49% was achieved at 3 years with 50 to 55 Gy in 15 to 16 fractions. Similar local control of 44% was reported in 25 patients treated with 8 Gy delivered on days 0, 7, and 21. A report on 31 patients from multiple institutes treated with definitive RT showed a local control of 58.1%. The authors concluded that hypofractionation with a dose per fraction greater than 3 Gy was associated with better local control and survival. Taking into account some preliminary results with particle radiation, a pooled analysis showed local control as high as 70% can be achieved with RT alone. Taken together, primary RT should be attempted for localized inoperable mucosal melanoma.
Definitive Radiation Therapy for Ocular Melanoma
Ocular melanoma is the most common primary intraocular malignant tumor in adults. Successful treatment of ocular melanoma with eye and vision preservation is one of the major triumphs of radiation oncology. Very high rates of local control can be achieved with particle radiation treatment or episcleral plaque BT.
Episcleral plaque brachytherapy for ocular melanoma
Initial experiences of episcleral BT used the high-energy isotope, 60 cobalt (60 Co). Since then, low-energy isotopes, such as 125 iodine (125 I), 192 iridium, 131 cesium, 103 protactinium, and 106 ruthenium/106 rhodium, have replaced 60 Co. 125 I is currently the most commonly used isotope. The Collaborative Ocular Melanoma Study conducted a 12-year study that demonstrated relative equivalence of 125 I plaque (85 Gy) compared with enucleation in the prevention of metastatic melanoma for medium-sized choroidal melanoma. Plaque BT was effective in sterilizing the gross tumor, with local control being achieved in approximately 90% of patients. However, radiation-induced ocular injury necessitated enucleation in approximately 5% of patients. Retrospective analyses suggest lower doses can achieve similar rates of disease control with lower rates of toxicities. Doses as low as 69 Gy may achieve similar rates of local control, distant metastasis-free survival, and overall survival as compared with 85 Gy. To minimize the potential of visual acuity loss, for tumors close to macula, a dose less than 70 Gy to the tumor apex should be considered.
Particle beam radiation therapy for ocular melanoma
Proton therapy is most commonly used for the treatment of ocular melanoma. It has the advantage over plaque therapy to treat larger tumors. For uveal melanoma, doses of 60 Gy delivered in 4 daily fractions of 15 Gy is highly effective. Actuarial 15-year local control rate is 95%, and eye preservation rate is 84%, based on an analysis of 2069 patients treated at Harvard Cyclotron laboratory and Proton Therapy Center at Massachusetts General Hospital between 1975 and 1997. A meta-analysis of 8809 patients with uveal melanoma included 7457 patients treated with charged particle therapy and 1352 patients with BT or enucleation. The rate of local recurrence was significantly lower with charged particle therapy than with BT (odds ratio 0.22). However, there were no significant differences in mortality or enucleation rates. Charged particle therapy was also associated with lower retinopathy and cataract formation rates. A prospective randomized trial of lower-dose (50 Gy) versus standard-dose (70 Gy) proton radiation for small-size to moderate-size uveal melanoma showed no differences in 5-year local or systemic recurrence or visual acuity loss.
Adjuvant radiation therapy for melanoma
Adjuvant Radiation Therapy for Primary Melanoma
The mainstay of treatment of cutaneous melanoma is surgery. Adjuvant radiation treatment can be used to reduce the risk of local-regional failure in patients who are at high risk of recurrence; this is based on retrospective studies and has not been defined by clinical trials. The common factors of high risk of local-regional recurrence include close positive margins, lymphatic space invasion, multiple recurrences, desmoplastic or neurotropic growth, extensive satellitosis, and mucosal melanoma. In these high-risk situations, adjuvant radiation treatment has a potential to reduce the risk of local-regional failure. Desmoplastic neurotropic growth is an unusual subtype of melanoma and is reported to have a high local recurrence rate of 20% to 50% after surgery. The benefit of adjuvant radiation is controversial. Recent analysis showed only 8% of such patients in the United States received RT. As a result, the North Central Cancer Center Treatment Group conducted a single-arm phase II trial to assess the role of adjuvant RT ( NCT00060333 ). The result has not been reported yet. There is also a current randomized trial comparing adjuvant RT (48 Gy in 20 fractions) to observation for patients with primary melanoma of the head and neck region with neurotropism ( NCT00975520 ). Results from these prospective studies would help define the role of adjuvant RT.
Adjuvant Radiation Therapy for Regional Lymphatic Metastases
Adjuvant radiation treatment after surgery decreases the rate of local recurrence for patients at high risk of regional failure after lymph node dissection; however, it does not improve overall survival. Risk factors for regional failure generally include multiple positive lymph nodes, large lymph node, extracapsular extension, and recurrence after prior lymph node dissection. Some of the largest retrospective series are from MD Anderson Cancer Center. The RT regimen is 30 Gy in 5 fractions over a period of 2.5 weeks. Local control is 94% for head and neck melanoma, 87% for axilla, and 74% for ilioinguinal disease. A prospective trial done in the 1970s was inconclusive. It has several significant problems, including small sample size (56 patients total, 27 in the adjuvant RT arm, and 29 in the surgery alone arm), inadequate RT (50 Gy in 28 fractions), and short follow-up time. The most meaningful data are from the phase III trial by the Australia and New Zealand Melanoma Trials Group and Trans-Tasman Radiation Oncology Group. Following surgery, 250 patients with positive lymph nodes deemed to be at high risk for locoregional recurrence were randomly assigned to RT (48 Gy in 20 fractions) or observation. Patients were considered at high risk if there was extracapsular extension, multiple positive nodes (≥1 for parotid, ≥2 for neck and axilla, and ≥3 for groin location), and large lymph node (≥3 cm for parotid, neck, and axilla, and ≥4 for groin location). After a median follow-up of 40 months, the radiation arm showed a significantly lower rate of lymph node recurrence as compared with observation. However, there were no differences in relapse-free survival or overall survival. An update presented at American Society of Clinical Oncology 2013 meeting with 6-year median follow-up confirmed these findings. The 5-year local recurrence rate was 18% for the RT arm and 33% for the observation arm ( P = .02), and no difference in relapse-free or overall survival.
Radiation therapy for distant metastasis
RT is most commonly used for palliation for melanoma distant metastasis. Palliative treatments are for the relief of symptoms, and RT is effective for pain, mass effect, tumor-related hemorrhage, local irritation from skin, or subcutaneous lesions. New RT techniques, such as SRS and SBRT, can achieve high probability of local control with very limited toxicity and are often preferred because of the relative radio-resistant nature of melanoma.
Brain metastases occur in more than 50% of patients with advanced melanoma. The median survival of these patients is 4.4 months and the 5-year survival rate is approximately 3%. Most of these patients die from central nervous system–related causes. Surgery, whole brain radiation therapy (WBRT), and SRS are all used in the treatment of brain metastasis; nonetheless, the best treatment remains controversial and many patients receive more than one modality. Radiation treatment is commonly used and effective. It results in symptomatic improvement and improved survival time. Melanoma is considered a less radiosensitive tumor, and the local control with WBRT is poor. The estimated local control rates with WBRT at 6 and 12 months are 37% and 15%. For patients with fewer metastases, SRS can be used as an alternative. SRS treatment significantly improved the local control rate of melanoma brain metastases compared with those treated with WBRT. The 12-month local control rate with SRS is about 65%. Local control for other metastatic diseases, such as bone/spine, lung, and liver, treated with SRS/SBRT is also very favorable (70%–90%). SBRT treatment also has the advantage of better pain control for bone metastasis. A large series of 500 patients (including melanoma) with spinal metastasis receiving single-fraction SRS treatment showed a long-term tumor control of 90%, and long-term pain control of 85%.
Aggressive local treatment with SRS or SBRT to the sites of metastases may be particularly meaningful in a clinical significant disease state of oligometastases. Multiple series indicate that 5-year survival can be as high as 15% to 41% in patients with a few sites of distant metastases that can be completely resected. Ablative radiation treatment with SRS or SBRT can be a particularly useful alternative. The University of Rochester reported 2 protocols of patients with 1 to 5 metastases (mainly breast, lung, and colon primary) treated with SBRT. The local control rate is 77% at 2 years. A similar experience from Duke University showed a 2-year local control rate of 52.7%, warranting evaluation in patients with metastatic melanoma.
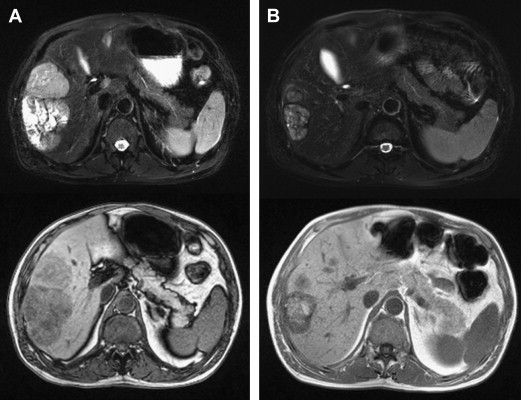
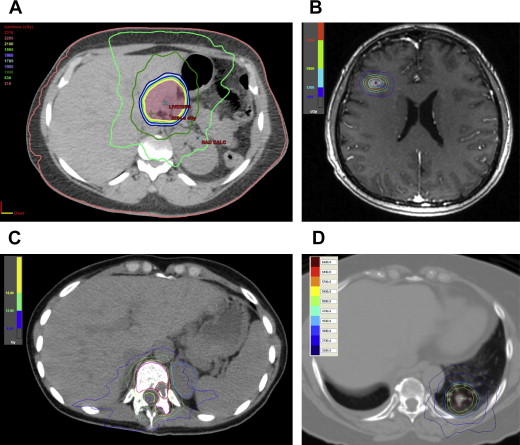
The liver is another common site for visceral melanoma metastasis and is involved in 15% to 20% of metastatic cutaneous melanoma, and up to 95% of metastatic ocular melanoma. Besides standard EBRT treatment, 90 Y radioembolization presents a new and attractive option for the management of liver metastasis. This radiation is a special form established for the treatment of hepatocellular carcinoma and liver metastasis. Existing experiences suggest it is an effective and safe option for managing hepatic metastasis from melanoma ( Fig. 2 ). Further studies will help establish its role and optimal patient selection.