Edward E. Walsh, Caroline Breese Hall *
Respiratory Syncytial Virus (RSV)
Were we but able to explain
The fiefdom of the microbe—
Why one man is his serf,
Another is his lord
When all are his domain.
C.B.H.
Respiratory syncytial virus (RSV) is the major cause of lower respiratory tract illness in young children.1–3,4 So effectively does RSV spread that essentially all persons have experienced RSV infection within the first few years of life. Immunity, however, is not complete, and reinfection is common. Although life-threatening infections most commonly occur during the first couple of years of life, RSV infections contribute an appreciable share of the morbidity caused by acute upper and lower respiratory tract infections among older children and adults. The health care costs associated with outpatient infections add appreciably to the estimated cost of $300 to $600 million for hospitalized infants with RSV infection.2,5,6 Of concern and increasing recognition are the growing morbidity and costs associated with RSV infections in older adults.7–9
History
RSV was discovered in 1956 but was not initially associated with respiratory illness among infants. Indeed, when a group of 14 chimpanzees were noted to be suffering from colds and coryza, Morris and co-workers10 isolated a new virus originally named chimpanzee coryza agent (CCA).1 Subsequently, Chanock and co-workers11confirmed that the agent caused respiratory illness in humans when they obtained isolates from two children, one with laryngotracheobronchitis and the other with bronchopneumonia, that were indistinguishable from CCA. When specific neutralizing antibody to CCA was found to be present in most school-aged children, “chimpanzee coryza agent” was more appropriately renamed respiratory syncytial virus to denote its clinical and laboratory manifestations.
Description
Classification
RSV belongs to the order Mononegavirales, family Paramyxoviridae, and the subfamily Pneumovirinae.12 The two genera within the Pneumovirinae subfamily are Metapneumovirus, containing human metapneumovirus (hMPV), and Pneumovirus, which contains human RSV and the morphologically and biologically similar murine pneumovirus and canine pneumovirus, bovine RSV, ovine RSV, and caprine RSV.13 Distinctive features of RSV include the number and order of genes and the lack of hemagglutinin and neuraminidase activity.
Viral Structure and Characteristics
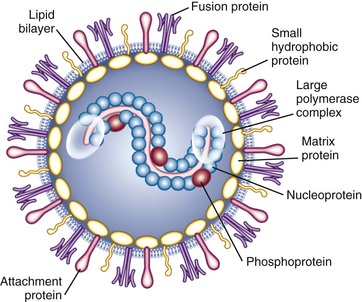
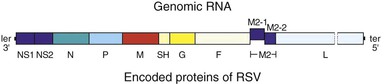
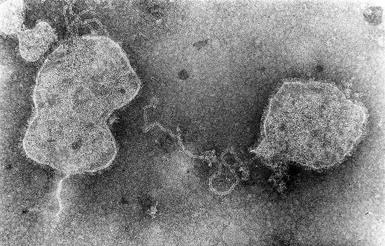
RSV is an enveloped, medium-sized (120 to 300 nm) RNA virus with a nonsegmented, single-stranded, negative-sense genome that is associated with viral proteins throughout its length, which form the nucleocapsid (Figs. 160-1 and 160-2).6,12 The viral envelope has a bilipid layer derived from the plasma membrane of the host cells. Its surface has transmembrane surface glycoprotein spikes 11 to 12 nm in length and 6 to 10 nm apart, which on electron microscopy give it a thistle-like appearance (Fig. 160-3).12
The complete genome of RSV (A2 strain) has been sequenced (see Fig. 160-2).12,14 The viral RNA consists of 15,222 nucleotides containing 10 genes. Each gene encodes for a single protein, except for the M2 gene, which possesses two overlapping open reading frames that encode for two separate proteins (M2-1 and M2-2, the transcription processivity factor and a transcriptional regulatory protein, respectively). Four proteins—N (nucleoprotein), P (phosphoprotein), L (polymerase), and M2-1—are associated with the RNA-containing nucleocapsid complex. Associated with the envelope are three transmembrane surface proteins, F (fusion), G (attachment), and SH (proposed viroporin protein), that are important for viral infectivity.15 In addition, a truncated secreted form of G (Gs) is transcribed from a second start codon. The M (matrix) protein accumulates at the inner surface of the envelope and is important in viral morphogenesis. Two proteins, NS1 and NS2, are nonstructural proteins that inhibit cellular type I interferon activity and subsequently effect the adaptive immune response to RSV.12,16
The two major glycosylated surface proteins, F and G, are the major immunoprotective antigens and are targets for antibody-mediated neutralization. The G protein is the primary mediator of attachment of the virus to host cells, although the F protein also can facilitate viral attachment.17 After attachment in the pretriggered form, F undergoes structural changes into a post-triggered form that initiates viral penetration by fusing viral and host cellular membranes and promotes fusion of infected cells to adjacent uninfected cells, thereby resulting in the characteristic RSV syncytia.18 Maximally efficient fusion requires participation of all three of the surface glycoproteins, F, G, and SH.
Laboratory Properties
RSV poorly withstands slow freezing and thawing and changes in pH. At 55° C, infectivity is rapidly diminished, and at room temperature (25° C), only 10% infectivity remains at 48 hours, and even at 4° C only 1% remains after 7 days.19 The optimal pH for RSV is 7.5. Inactivation occurs quickly with acidic media (pH < 5), ether, and chloroform and with detergents such as sodium dodecyl sulfate and Triton X-100.
The survival of RSV in the environment appears to depend in part on the drying time and dew point. At room temperature, RSV in the secretions of patients may survive on nonporous surfaces, such as countertops, for 3 to 30 hours.20 On porous surfaces, such as cloth and paper tissue, survival is generally shorter, usually less than 1 hour. The infectivity of RSV on the hands is variable from person to person but is usually less than 1 hour.
Human heteroploid cell lines are usually preferred for primary isolation. Most commonly used are HEp-2, HeLa, and A549 cell lines. RSV may also be recovered in human kidney, amnion, diploid fibroblastic cells, and monkey kidney cells. With primary isolation in sensitive cell cultures, characteristic cytopathic effect may be first detected after an average of 3 to 5 days. The typical syncytia develop about 10 to 24 hours later and progress until the cell sheet is completely destroyed, which is usually within 4 days. Cell-free virus may be demonstrated in the culture medium, but up to 90% of the virus remains cell-associated.12
Infection in Animals
Humans and chimpanzees are the only known natural hosts for human RSV, although a variety of small animal species may be experimentally infected with RSV.21 Cows are the natural hosts of bovine RSV (BRSV), which is antigenically and genetically closely related to human RSV, and some antibodies directed against the F, N, P, and M proteins of either virus recognize the heterologous virus. Ovine and caprine strains of RSV also have been recovered, and genetic analysis suggests that caprine RSV is more closely related than ovine RSV to BRSV and human RSV.12
Although many animal models develop upper respiratory tract infection, their lack of symptomatic lower respiratory tract disease comparable to that observed with infants limits their utility. Closest is disease in chimpanzees because they readily acquire infection from infected contacts and shed moderate levels of RSV in respiratory secretions.12 Nevertheless, their disease is generally mild and without the degree of lower respiratory tract involvement observed in infants. Rodents are the most commonly used models, particularly cotton rats and mice, but replication of RSV is only semipermissive and highly variable.22
Epidemiology
Distribution
In every geographic area studied, RSV infections are ubiquitous and clinically similar in causing the most severe disease during infancy and repetitive infections throughout life.6,23,24 The seasonal occurrence, however, varies according to geography and climate.25
Seasonal Occurrence
RSV is singular in its ability to produce reliably a major burden of infections every year.3,26 In temperate climates, the outbreaks occur primarily in the late fall through early spring and spread across the United States over a period of 20 or more weeks, generally from October to May. In warmer climates, RSV activity may be more prolonged or even present throughout the year.24,25,27–29 What factors initiate and terminate the recurring patterns of RSV activity has been a subject of ongoing curiosity and investigation but remains a conundrum.29–31 A complex, and currently incompletely understood, interaction of local meteorologic conditions may explain part of the geographically variable epidemiologic patterns.32 Among these are the ambient temperature, humidity, and sunlight, and their effects on RSV’s stability and infectivity. However, because the only source of RSV infection is an infected individual, human behavior is an indefinable factor integral to its transmission.29,33
Antigenic Variation
Strain differences among RSV isolates may also affect the intensity, severity, and diversity of RSV outbreaks.34–36 RSV isolates are divided into two major antigenic groups, A and B, each of which can be further divided into multiple subgroups containing separate genotypes.12,14,37 The two major groups have 81% nucleotide identity, but some of the proteins between the A and B strains vary appreciably. The major genetic diversity between groups A and B resides with the G protein, followed by M2-2 and SH proteins. This is reflected in the relative low antigenic relatedness between the two groups of only 1% to 7% for the G proteins, compared with 50% for the F proteins. Nucleotide and amino acid sequence analysis within both A and B strain groups has further revealed subtypes of distinctive lineage, or clades.12,14
Strains of both groups circulate simultaneously during outbreaks, but the proportions that are A and B vary, as do the subtypes.34–38 Even in widely separated geographic areas, the cocirculating strains may have similar genotypes and parallel evolutionary lineages. Analyses of the proteins of strains collected over decades and from diverse areas suggest that the pressure of the population’s immunity may result in a selective advantage for the dominance of strains that are most divergent from those that have recently circulated in the area. Evidence indicates that a recently emerged RSV B strain containing a unique 20-amino-acid duplication in the G protein has spread throughout the world and currently is the dominant variant among group B viruses, suggesting an evolutionary advantage over prior strains.39 However, the relationship of the circulating strain groups and subtypes to the clinical severity and manifestations of the RSV infections among young children has been inconsistent and thus inconclusive.35,36,38,40,41 The relationship between the substantial antigenic diversity of the G protein to reinfection with RSV has not been established.
Epidemiologic Manifestations
The characteristic epidemiologic ramifications of RSV’s presence within a community are notable rises in the number of cases of bronchiolitis, pneumonia, and hospital admissions of young children, especially infants with acute lower respiratory tract disease.2,42,43 RSV outbreaks may vary year to year in size and intensity. Severe lower respiratory tract illness from RSV in previously healthy children occurs most frequently in the first year of life and is almost always associated with primary infection. Essentially all children have experienced RSV infection within the first several years of life one or more times. Repeated infections are common not only among young children but throughout life.7,44–46
Prevalence and Incidence
RSV is the most frequent cause of bronchiolitis and is estimated to cause 40% to 90% of bronchiolitis hospitalizations and up to 50% of pneumonia admissions among infants.2,3,5,43 Ten to 30% of tracheobronchitis cases have been associated with RSV infection, but only 2% to 10% of croup cases. Bronchiolitis is the leading cause of all hospitalizations among infants in the United States. Overall, as many as 172,000 children younger than 5 years are reported to be hospitalized annually with RSV infection in the United States.3,4,47 The yearly rates of RSV hospitalizations estimated from national databases have been variable, ranging from about 2 to 44 per 1000 children within the first 2 to 5 years of life.42,48,49 Children within the first year of life consistently had the highest rates of hospitalization for bronchiolitis and other RSV-associated illnesses, and the preponderance of admissions were among infants younger than 6 months.4 Recent population-based studies in the United States have indicated the current hospitalization rates are 17 per 1000 children younger than 6 months and 3 per 1000 children younger than 5 years.2,43 These rates, which are similar to those derived from RSV-coded hospitalization data, were more than three times the rates from parainfluenza or influenza viral infections over 4 years of surveillance among the same population of children.50,51 The reported RSV hospitalization rates from European countries have been generally similar, ranging from 2.5 to 11 per 1000 children within the first 4 years of life and highest among those younger than 12 months of age—19 to 22 per 1000 children.5,52–54 Estimates of lower respiratory tract infection from RSV in developing countries are estimated to be twice that of developed countries.27
The rates of hospitalization among children, however, vary according to the presence of risk factors. Many host, socioeconomic, and environmental factors have been associated with a greater likelihood of young children developing more severe RSV infection and requiring hospitalization (see “Patients at High Risk for Severe Infection”).
Population-based surveillance during 2000 to 2004, examining laboratory-proven RSV hospitalizations and emergency department and outpatient visits in the counties surrounding Rochester, New York; Nashville, Tennessee; and Cincinnati, Ohio, indicated that outpatient visits constituted a major proportion of the health care burden attributable to RSV infection.2 Of all visits for acute respiratory illnesses (ARI) among children within the first 5 years of life, RSV infection was documented among 20% of ARI hospitalizations, 18% of emergency department ARI visits, and 15% of office ARI visits. The estimated rates of ARI visits from RSV among children younger than 5 years in pediatric practices (80 per 1000 children) was about 3 times higher than that observed among emergency department patients and 26 times the rates among hospitalized children.
Less information exists on the health care burden imposed by RSV infection among older children and adults. Recurrent RSV infections are common among school-aged children and adults who are exposed to RSV at school, home, work, or during military training.49,55 In urban Rochester, 44% of the families with young children who were followed became infected with RSV during the winter months, when RSV was prevalent in the community.46 Of the exposed family members, 46% acquired RSV infection. Although the attack rate was highest among infants, 38% to 47% of older children and adults developed RSV infection. In the Houston family study, in which children were followed from birth, the infection rate was 68.8 per 100 children in the first year of life, and during the second year, at least half were reinfected.44
More recently recognized is the frequency of RSV infections among older adults and the resulting appreciable clinical and economic impact.7,9,56 In one study spanning 4 years, rates of RSV infection ranged from 2% to 10% per year in persons 65 years of age or older and in high-risk persons with underlying cardiopulmonary disease.7 RSV infection among older individuals is remarkably similar to influenza with respect to clinical manifestations and as a cause of hospitalization. In London, the rate of hospitalization attributable to RSV infection among the elderly has been estimated at 0.7 per 1000 adults 65 years of age or older, compared with 1.1 per 1000 estimated for influenza.57
Pathogenesis
Infection with RSV is primarily acquired through close contact with an infected individual or direct inoculation into the eyes and nose of infectious secretions.33,58 Large-particle aerosols engendered by coughs and sneezes of an ill person may transmit RSV to others within a radius of about 3 feet. Longer-distance spread by small-particle (droplet nuclei) aerosols appears to be much less likely.33 However, infection occurring from touching objects that have been contaminated with infectious secretions, followed by self-inoculation into the eyes or nose, is an important mode of transmission.
Experimental infection occurs in adult volunteers after an average incubation period of 3 to 5 days.59–61,62, 63 In naturally acquired infection, the average incubation period appears similar, with a range of 2 to 8 days. The mucosa of the nose and eye appears to be equally sensitive portals of entry, in contrast to the mouth.61 In hospitalized infants with primary RSV infection, peak viral titers range from 101 to 107 plaque-forming units (pfu)/mL of nasal secretions (mean of 105) and fall slowly.64,65 Shedding duration is typically 7 to 10 days, but virus can occasionally be detected as long as 30 days later. Increased viral load has variably been associated with greater disease severity.64–66
RSV replicates in respiratory epithelium, primarily involving the ciliated columnar cells, but additional cells, such as type I and II pneumocytes, may be involved.67 During primary infection, lower respiratory tract infection usually is manifest by bronchiolitis, and the initial pathologic findings are a lymphocytic peribronchiolar infiltration, predominantly CD69+ monocytes, with edema of the walls and surrounding tissue.67–69 Subsequently, the characteristic proliferation and necrosis of the epithelium of the bronchioles develop. The lumina of these small airways become obstructed from the sloughed epithelium and from the increased mucus secretion. The airway of the young infant is particularly vulnerable to any degree of inflammation and obstruction because resistance to the flow of air is related inversely to the cube of the radius. Impedance to the flow of air occurs during both inspiration and expiration but is greater in the latter when the lumen is narrowed further by the positive expiratory pressure. Hyperinflation, therefore, results from air trapping peripheral to the sites of partial occlusion. With complete obstruction, the trapped air eventually becomes absorbed, producing the characteristic multiple areas of atelectasis. Young infants are at increased risk for developing such areas of atelectasis because the collateral channels that maintain alveolar expansion in the presence of airway obstruction are not yet well developed. These changes result in an increase in lung volume and expiratory resistance.
Infants with lower respiratory tract disease from RSV often have pathologic evidence of both pneumonia and bronchiolitis. Patients with pneumonia demonstrate an interstitial infiltration of mononuclear cells that may be accompanied by edema and necrotic areas that lead to alveolar filling.67,68 Some histologic evidence of recovery is present in most children with bronchiolitis within the first week of illness and is marked by the beginning regeneration of the bronchiolar epithelium. However, ciliated cells may not be present for weeks.
Immunity and Pathogenesis of Disease
Diverse theories and supporting data have been offered to explain how RSV engenders these pathogenic findings. Much of our current knowledge regarding the immunologic responses to RSV is from studies in vitro and in animal models. In humans, the immune response cannot be separated from the confounding and poorly understood influence of genetics, environment, age, and antigenic experience.70–73 Consensus exists that during primary infection, disease is both virally and immunologically mediated. RSV produces its most devastating illness when specific, maternally derived antibody is present at variable amounts. The severity of RSV infection in the young infant with high levels of specific antibody and the augmented disease induced by the inactivated RSV vaccine developed in the 1960s first suggested the putative singular role of the immune response in RSV’s pathogenesis in infants.73–77
The potential importance of the host’s immune response to the development of disease and the subsequent rowen of long-term complications has been further supported by the observation that RSV is not very invasive or cytopathogenic.73 Although viral loads correlate with disease severity in humans, reducing viral replication with the administration of neutralizing antibody to the F protein has not ameliorated clinical disease in infants.78,79 However, recent data in murine models of RSV indicate that administration of non-neutralizing antibodies to the centrally conserved CX3C chemokine motif of the G protein can reduce inflammatory responses and illness even after infection is established.80,81
The relative contribution of the immune response to disease in infants, however, has been questioned by the observation that among children with bronchiolitis, those with RSV bronchiolitis produced a more robust response of proinflammatory cytokines in their nasal washes than did children with non-RSV bronchiolitis, but it was not associated with more severe disease and, indeed, appeared protective against hypoxia.82 Furthermore, examination of fatal RSV cases has shown that RSV antigen was extensively present in the pulmonary tissue, indicating abundant viral replication.83 Cytokine production, on the other hand, was nearly absent, and the expression of apoptosis was increased, with the conclusion that the patients had an inadequate immune response and unchecked viral replication. Whether the innate or adaptive immune responses, or both, are enhanced or suppressed in association with more severe disease remains controversial, but most likely the pathogenicity of RSV infection results from varying and currently ill-defined combinations of the contributions of the virus and host.
Innate Immunity
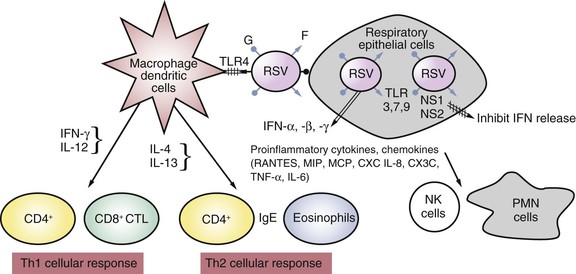
The first barrier of defense against RSV infection in infants is the innate immune response, which is initiated by infection of the respiratory epithelium.73,77,84,85 RSV is a potent inhibitor of cellular antiviral type I interferons (α, β, and λ) via the production of the NS1 and NS2 proteins.16 In addition, RSV interacts with the Toll-like receptor (TLR)2, 3, 4, and 7, which triggers secretion of inflammatory cytokines.77 Infection of the respiratory epithelium, antigen-processing cells (dendrites), and macrophages produces multiple alterations in gene expression, which result in the production of cell surface markers and the release of cytokines and chemokines (Fig. 160-4). Inflammatory cells are recruited to the respiratory tract and consist of neutrophils as well as macrophages, mononuclear cells, T cells, natural killer (NK) cells, and eosinophils. Intubated infants with severe primary RSV infection have an early and robust neutrophilic infiltration into the airway, as determined by bronchoalveoloar lavage, and this correlated with a decline in viral load before the development of T-cell responses.86 The variability in the endowed innate defense and susceptibility of the host are also being increasingly correlated with polymorphisms in genes that are integral to various components of innate immunity.72,87
Adaptive Immunity
The relative contributions and interactions of different arms of the immune response to either a primary or recurrent exposure to RSV or after immunization with experimental vaccines are complex and unclear. Considerable evidence suggests that an effective, nondetrimental immune response to RSV infection requires a fine balance of the multiple components of immunity, and that balance is determined by both host and viral factors.70,73
Serum neutralizing antibody provides some, but not complete, protection against RSV infection. Higher titers of antibody generally correlate with better resistance to infection, but no defined level of neutralizing antibody is predictive of the risk for infection, the severity of illness, or recovery in children or adults.8,88–90 Higher levels of maternal antibody have been correlated with lower infection rates and with less severe illness in some studies, but not in others.91,92 More recently, the trials of administration of RSV monoclonal antibody to high-risk infants have demonstrated protection against more severe RSV disease.93
Passively derived maternal antibody usually declines to undetectable levels by 6 months of age, but occasionally, it may remain up to 9 to 12 months of age. During primary infection, serum immunoglobulin M (IgM) antibody appears within several days, but it is transient and detectable usually only for a few weeks.73 During the second week, IgG antibody appears, usually peaks in the fourth week, and begins to decline after 1 to 2 months. The IgA serum antibody response is more variable in infants. An anamnestic response involving all three immunoglobulin classes occurs after reinfection, and after about three infections, the titers reach levels similar to those in adults.
Primary and subsequent infection produce antibody to many of the RSV proteins. However, the major immunoprotective antigens are two large surface glycoproteins, F and G.12 Both contain neutralizing epitopes, but those on the F protein are conserved between the two strain groups, resulting in antibody to the F protein being cross-reactive between group A and B strains. In contrast, the response to the variable G protein is group and genotype specific.94 Most adults with RSV infection develop IgG responses to the centrally conserved chemokine motif of G, although their role in recovery or protection from infection or illness is not clear.95
Although young children are able to produce neutralizing antibodies directed against both the F and G proteins, the neutralizing antibody responses are blunted in infants younger than 6 months because of a dampening effect of maternal antibody.96 In infants, the antibody response to the F and G proteins mainly involves the subclasses IgG1 and IgG3. However, adults respond to the G protein with antibodies in both the IgG1 and the IgG2 subclasses, and the adult response to the F protein is predominantly IgG1. Antibodies after primary and recurrent infection usually decline substantially within months. Subsequent to natural infection, 75% of adults demonstrate a fourfold or greater drop in titer, returning to preinfection titers within 2 years in most.97 A protective role for local antibody in RSV infection has been suggested because animal studies indicate that circulating IgG does not prevent viral replication in the nasal passages.90,98 RSV-specific IgA antibody, which is produced in nasal secretions during primary and subsequent infection, has been associated with protecting the upper respiratory tract from infection in adults and has been correlated with clearance of viral shedding in infants.88,90,99 Children with RSV infection may also produce transient specific IgE antibody responses in the respiratory tract. Levels in the nasal secretions of both RSV-specific IgE antibodies and of cysteinyl leukotrienes have been correlated with increased risk for more severe acute infection, with wheezing and with later episodes of recurrent wheezing.100,101
Cell-mediated immunity is likely to be pivotal in the clearance of virus and in recovery, but has not been definitively shown to protect against reinfection and illness in humans. Adults and children with deficiencies of cellular immunity, as well as experimentally immunosuppressed animals, have more severe disease and prolonged virus shedding.102,103 Most information delineating the specific components of the cellular response induced by RSV are derived from rodent models and, to a much lesser extent, from humans.70,73
RSV infection has multiple suppressive effects on the cellular immune response. Diminished in vitro lymphoproliferative responses during initial and repeated infections suggest impaired RSV-specific helper T-cell responses. Furthermore, RSV-infected dendritic cells have diminished ability to activate CD4+ T cells, and enhanced apoptosis of CD4+ and CD8+ lymphocytes is observed among infants with RSV bronchiolitis.104,105
RSV infection in both animals and humans engenders a spectrum of both Th1- and Th2-dominant responses (see Fig. 160-4). Th1-dominant responses, characterized by the production of CD8+ cytotoxic lymphocyte and Th1 CD4+ cells secreting interleukin-2 (IL-2), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) are associated with viral clearance and minimal pulmonary cytopathology. In contrast, Th2-dominant responses induce Th2 CD4+ cells, which stimulate IL-4, IL-5, IL-10, and IL-13 secretion. These Th2 cytokines affect CD8+ T-cell function and impair viral clearance.22 IL-4 and IL-13 also augment isotype switching to IgE, and a Th2-biased response has been correlated with wheezing, more severe disease, and greater cellular inflammation and eosinophilia in the lung.100
Whether RSV evokes predominantly a Th1- or Th2-biased response has been shown to be influenced by the individual’s genetic background, age, and previous RSV experience and the specific antigen. Infants, within the first several months of life, have been observed to have higher levels of Th2-type cytokines in nasal secretions than older infants.106 Inactivated virus, as used in the initial formalin-inactivated vaccine and even in subunit vaccines, is more likely to induce a Th2-like response in experiments with unprimed animals than is live virus.22 Antigenic stimulation with the F protein is associated with a predominantly Th1 response, whereas the G protein produces a Th2-biased response, possibly because its CX3C motif adversely affects the CD8+ T-cell response.107
Clinical Manifestations
Clinical observations over the half century of RSV’s recognition have confirmed the important immunologic correlates that naturally acquired immunity to RSV infection is incomplete, variable, and not durable. Repeated infections are common, but severe disease rarely occurs after the primary encounter in normal healthy children. Lower respiratory tract involvement and severe disease may occur during repeat infections, but it is generally confined to those at either end of the age spectrum.7,44,45
Infection among Young Children
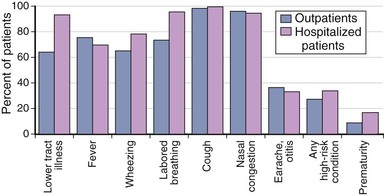
Primary infections with RSV frequently involve the lower respiratory tract, particularly among infants within the first several months of life. Most commonly, these present as bronchiolitis, followed by pneumonia and tracheobronchitis.2,44,45 Croup is the least common form of clinical illness and usually accounts for less than 2% to 10% of cases. Upper respiratory tract signs almost always accompany lower respiratory tract disease, or the infection may be confined to the upper respiratory tract, which in young children is commonly associated with fever and otitis media (Fig. 160-5). Rarely is the first infection asymptomatic.44–46 The risk for lower respiratory tract involvement occurring with the first infection is high. Pneumonia or bronchiolitis has been estimated to occur in 30% to 71%, depending on the age and population.2,44,45,108 Among infants younger than 6 months, those with underlying cardiopulmonary disease, and those in close contact with other young children, such as those attending daycare, the proportion developing lower respiratory tract disease may be even higher.45 Furthermore, the severity of illness among outpatient preschool-aged children is considerable, with two thirds manifesting wheezing and three fourths developing labored breathing (see Fig. 160-5).
RSV infection among young children and older individuals usually starts with an upper respiratory tract illness with nasal congestion and a cough. Hoarseness and laryngitis are not prominent features. A low-grade fever, lasting for 2 to 4 days, occurs in most young children early in the course of the illness. The height or duration of the fever does not correlate with the severity of the disease and is frequently absent in the presence of lower respiratory tract involvement and at the time of hospitalization. With progression of disease to the lower respiratory tract, the cough may become more prominent and productive, followed by an increased respiratory rate, dyspnea, and retractions of the intercostal muscles. With bronchiolitis, both expiratory and inspiratory obstruction may be evident. On auscultation, the infant may have crackles and wheezing. The rapid variability in the presence and intensity of these physical findings is characteristic of bronchiolitis, with marked transient swings in oxygen saturation often noted. Repeated observations of the infant, therefore, are required for adequate assessment of clinical severity.109
Infants hospitalized with RSV lower respiratory tract infection commonly have some impairment of oxygenation.109 This reflects diffuse viral involvement of the lung parenchyma, which causes an abnormally low ratio of ventilation to perfusion. Usually, this is not clinically apparent, and mild degrees of desaturation may persist despite clinical improvement. Approximately 10% of hospitalized infants will develop alveolar hypoventilation and progressive hypercarbia and may require assisted ventilation. Hospitalization, if required, averages 3 days, but prolonged hospitalization is not uncommon.2 For most infants, however, the duration of the acute illness is 3 to 10 days, but respiratory signs, especially cough, may be present for 4 or more weeks.
The abnormalities observed on chest radiograph may be minimal, irrespective of the severity of the child’s illness. Hyperaeration has been shown to be especially indicative of RSV infection and may be associated with peribronchial thickening.110,111 Most children exhibit only airway disease. Less than 10% of bronchiolitis cases have evidence of both airway and airspace disease, and only about 1% have parenchymal consolidation.110 However, about 20% to 35% of children with RSV lower respiratory tract disease have opacities, which are commonly misdiagnosed as bacterial pneumonia. These most commonly are subsegmental in the right upper or middle lobe and result from atelectasis. Pleural fluid is rarely demonstrated.
Otitis Media
Acute otitis media is a common complication of RSV infection among young children.112–114 Among children within the first 3 years of life who developed acute otitis media, as many as 74% have had RSV detected in their middle ear fluid. Acute otitis media usually develops about 5 days after the onset of the respiratory illness and is more common among children older than 1 year than in infants. Even among older children and previously healthy adults with RSV infection, otitis and earache are common complications, noted in approximately half of hospitalized infants in one study.46,55,114 RSV has been recovered from the middle ear fluid as the sole pathogen, with bacteria, or with another virus in up 2% to 22% of cases.112,114 RSV increases adherence of bacteria to respiratory epithelial cells, and the most frequent concurrent bacterial pathogens are those commonly found in the respiratory tract, especially Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis.114 Clinical and experimental evidence suggests that coinfection of RSV with a bacterial pathogen may prolong the duration and worsen the outcome of otitis media, resulting in a greater chance of treatment failure with antibiotics and persistent effusion.
Infections among Older Children
The frequency of RSV infections at all ages is well illustrated among families with young children and among those in contact with young children, as in schools and daycare facilities.44–46 Among longitudinally followed children attending daycare facilities who have had primary RSV infection in their first winter, 75% and 65% develop infection during their second and third years, respectively.45 Recurrent infections commonly are upper respiratory tract illnesses, but 20% to 50% of recurrent infections among preschool-aged children involve the lower respiratory tract.44 Although second episodes of lower respiratory tract illness among children are rarely as severe as the initial episode, many are associated with wheezing. The overall burden of repeated infection in children younger than 5 years is substantial.2
Infections among Adults
Adults also may have repetitive RSV infection occurring within sequential years, especially those living or working with children or in confined military and university settings.46,55,58,115,116 The lack of durable immunity evoked by natural infection is illustrated by a study of 15 healthy adults who became ill with a community-acquired RSV A strain and were subsequently challenged with an RSV A strain at repetitive intervals over 2 to 26 months.88 Within 2 months of their natural infection, 47% became reinfected, and by 8 months, two thirds had been reinfected. However, successive infections, especially those occurring within close intervals, were associated with decreasing symptoms and shedding. Nevertheless, observational studies have noted that both infection and severity of RSV infection in adults are reduced by higher titers of serum and mucosal antibody.8,90
The extent of the burden RSV places on the health care of this growing population has only recently been appreciated fully.3,7,9,56,117–119 Those at greatest risk of severe illness include the frail elderly; those with underlying cardiopulmonary disease, especially chronic obstructive pulmonary disease (COPD); and the severely immunocompromised. Adults, even those with severe disease, shed RSV at titers considerably lower than infants, and thus estimates of the incidence of RSV infection in this population have been hampered until recently by the lack of sensitive diagnostic tests. The incidence of RSV hospitalization, found by using a regression model incorporating viral culture data from infants and hospital coding data from adults, has been estimated at 86 per 100,000, or 28% of the incidence of influenza hospitalizations.3 Using similar methods from the Netherlands to model data, RSV mortality was 64% of that attributable to influenza.9 Others have noted similar hospitalization and mortality rates in comparison to influenza.120–122
In long-term care facilities, 5% to 27% of respiratory infections have been reported to be caused by RSV. The attack rate generally has been estimated as 1% to 15%, the rate of pneumonia as 10% to 20%, and the mortality as 2% to 5%.117 A retrospective cohort analysis estimated that among Tennessee nursing home patients, RSV was associated with 15 hospitalizations, 17 deaths, and 76 antibiotic courses per 1000 people, and 7% of hospitalizations and 9% of deaths from cardiopulmonary disease.122 In a study of older adults admitted with cardiopulmonary conditions, 10% had RSV infection, compared with 13% with influenza.119 Illness was equally severe among the two groups: 18% required intensive care, and 10% of the RSV patients died, compared with 6% of the influenza patients. Similarly, among a prospectively studied population of older adults living in the community, RSV was identified as the cause of 7% and influenza as the cause of 9% of respiratory illnesses with identifiable pathogens.123 The morbidity associated with the two viruses was also comparable, with 82% of the RSV patients and 79% of the influenza patients developing lower respiratory tract disease. Among adults attending daycare facilities, 10% of acute respiratory infections were found to be caused by RSV, a rate similar to the rates for influenza and coronaviruses.124
The highest risk for severe RSV infection is in persons with underlying cardiopulmonary disease, especially COPD and congestive heart failure.7,56,119,125–127 A 4-year study of respiratory illnesses among prospectively followed elderly and high-risk adults living in the community emphasizes the epidemiologic and clinical impact of RSV infection among these populations (Table 160-1).7 In this study, 608 healthy persons age 65 and older and 504 high-risk people with cardiopulmonary conditions were evaluated for RSV infection by culture, reverse-transcriptase polymerase chain reaction (RT-PCR), and serology. A third group of 1388 adults hospitalized with acute cardiopulmonary conditions was also evaluated. During 4 years, RSV infection was identified in 3% to 7% of the elderly cohort, 4% to 10% of the high-risk cohort, and 10% of the hospitalized group. In comparison, influenza infection was identified among 4% of the individuals in the prospectively followed cohorts and among 11% of the hospitalized patients. Clinically, the illnesses from RSV and influenza were remarkably similar. Among the healthy elderly outpatients, those with RSV infection and those with influenza were ill for an average of 16 days. Of those with RSV infection, 39% were unable to perform their activities of daily living, only slightly less than with influenza. Among the hospitalized RSV patients, intensive care was required for 15%, and the mortality rate was 8%, compared with 12% and 7%, respectively, among influenza patients. RSV was identified in 5.4% of admissions for congestive heart failure, 11.4% of COPD exacerbations, and 10.6% of pneumonia diagnoses. Among the prospective cohorts, almost all RSV infections were symptomatic and frequently associated with appreciable functional impairment and use of health care services. The clinical illness caused by RSV in elderly persons is nonspecific and not readily distinguishable from other common respiratory viruses.118 Typical upper respiratory symptoms are more frequent than with influenza and precede lower respiratory symptoms by several days. Wheezing is more common with RSV infection, especially in patients with COPD, and chest radiographic findings are often minimal.
TABLE 160-1
Respiratory Syncytial Virus (RSV) Compared with Influenza among Hospitalized Adults*
| CHARACTERISTICS | RSV (N = 132) | Influenza A (N = 144) |
| Age (yr) | 76 ± 13 | 76 ± 12 |
| Female sex—N (%) | 84 (64) | 81 (56) |
| Chronic illness—N (%) | ||
| Any cardiac disease | 71 (54) | 71 (49) |
| Congestive heart failure | 39 (30) | 33 (23) |
| Any lung disease | 77 (58) | 79 (55) |
| Any heart or lung disease | 106 (80) | 113 (78) |
| Diabetes mellitus | 35 (27) | 28 (19) |
| Residence in a long-term care facility—N (%) | 16 (12) | 15 (10) |
| Smoking (current or past)—N (%) | 88 (67) | 98 (68) |
| Influenza vaccination—N (%) | 99 (75) | 98 (68) |
| Katz ADL score—mean ± SD | 1.2 ± 2.4 | 1.3 ± 3.0 |
| IADL score—mean ± SD | 4.1 ± 4.1 | 3.3 ± 4.0 |
| Length of hospital stay—days | 14 ± 41 | 8 ± 5 |
| Findings on chest radiography—N (%) | ||
| Infiltrate found | 41 (31) | 43 (30) |
| Congestive heart failure | 17 (13) | 15 (10) |
| Other | 24 (18) | 27 (19) |
| Admission to intensive care unit—N (%) | 20 (15) | 17 (12) |
| Use of mechanical ventilation—N (%) | 17 (13) | 15 (10) |
| Higher level of care at discharge than at admission—N (%) | 7 (5) | 8 (6) |
| Death—N (%) | 10 (8) | 10 (7) |
* Plus-minus values are means plus or minus standard deviation. The Katz Activities of Daily Living (ADL) score and the Instrumental Activities of Daily Living (IADL) score are functional assessments based on a 12-point scale, with 0 representing total independence and 12 representing total dependence. Percentage may not sum to 100 because of rounding.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree