Respiratory complications
Vickie R. Shannon, MD  George A. Eapen, MD
George A. Eapen, MD  Carlos A. Jimenez, MD
Carlos A. Jimenez, MD  Horiana B. Grosu, MD, Rodolfo C. Morice, MD
Horiana B. Grosu, MD, Rodolfo C. Morice, MD  Lara Bashoura, MD
Lara Bashoura, MD  Scott E. Evans, MD
Scott E. Evans, MD  Roberto Adachi, MD
Roberto Adachi, MD  Michael Kroll, MD
Michael Kroll, MD  Saadia A. Faiz, MD
Saadia A. Faiz, MD  Diwakar D. Balachandran, MD
Diwakar D. Balachandran, MD  Selvaraj E. Pravinkumar, MD, FRCP
Selvaraj E. Pravinkumar, MD, FRCP  Burton F. Dickey, MD
Burton F. Dickey, MD
Overview
The respiratory system is particularly susceptible to complications of cancer and cancer therapy. This vulnerability arises from the stringent architectural requirements for gas exchange, the continuous exposure of the respiratory tract to the external environment, and the severe symptoms that can accompany respiratory compromise. Gas exchange requires patent airways, an effective musculoskeletal ventilatory pump, a thin alveolocapillary membrane, and adequate blood flow through the pulmonary circulation. In cancer patients, primary and metastatic tumors of the chest compromise major airways; pleural effusions externally compress the lungs and impair diaphragmatic function; direct, hematogenous, or lymphangitic spread of tumor replaces functioning lung parenchyma; resectional surgery reduces parenchymal volume; nonresectional surgery can transiently impair lung function; radiotherapy, chemotherapy, stem cell therapy, and infection injure the vulnerable alveolocapillary membrane; tumors directly or indirectly compromise the musculoskeletal pump; and venous thromboembolism (VTE) and pulmonary vasculopathy obstruct pulmonary blood flow.
The normal respiratory system contains considerable physiologic reserve, such that surgical loss of one lung is generally well tolerated. However, in cancer patients, insults to multiple components of the respiratory system may result in progressive loss of physiologic reserve and increasing dyspnea. Dyspnea, cough, wheezing, stridor, chest pain, and hemoptysis are common symptoms in the cancer setting that lead to pulmonary consultation.
In this chapter, we will discuss the pathophysiology, diagnosis, and management of the major respiratory complications of cancer and its therapy. We begin with the direct effects of cancer and cancer therapies on the lungs, review major indirect effects of cancer on the lungs, and end with respiratory failure in the cancer patient.
Malignant airway obstruction
Malignant airspace disease may be central or peripheral, focal or diffuse, or endoluminal or extraluminal or both. The dominant symptom complex associated with this process depends upon the location and extent of disease, which also dictates therapy.
Common cancer types and clinical presentation
The most common cause of malignant airway obstruction is direct extension from an adjacent tumor, particularly bronchogenic carcinoma. Esophageal and thyroid malignancies also frequently extend directly into the airways. Primary tumors of the major airways are relatively rare, with squamous cell carcinoma, adenoid cystic carcinoma, and carcinoid tumors most often implicated.1, 2 Airway compromise can also occur from metastatic renal and breast carcinomas or intrathoracic lymphomas. Both endoluminal disease and extrinsic compression by tumor may severely compromise airway luminal diameter. Reduction in airway caliber and architectural distortion synergistically impair airflow obstruction and mucus clearance, leading to increased work of breathing and dyspnea.3 Luminal narrowing of the trachea and mainstem bronchi typically manifests as dyspnea, cough, wheeze, stridor, and atelectasis. Airway obstruction beyond the mainstem bronchi usually results in atelectasis, postobstructive pneumonitis, cough, and dyspnea. Individual patient presentations range from asymptomatic discovery on staging work-up to frank respiratory failure due to critical airway obstruction.4 Exertional dyspnea typically occurs when tracheal diameter is reduced below 8 mm. Further reduction in tracheal diameter to <5 mm is usually associated with dyspnea at rest.5 Chronic obstructive pulmonary disease (COPD) exacerbations or mucosal edema and increased secretions that accompany superimposed pneumonias may precipitate respiratory failure, even in patients with only moderate tumor-related airflow limitation. Symptoms may thus improve with measures directed at treating the infection or COPD exacerbation.
Differential diagnosis
While critical airway obstruction is not usually a diagnostic challenge, the clinical presentation of subcritical obstruction can be. Stridor indicates pathognomonic significant tracheal obstruction. Other findings, including dyspnea and wheezing, are prominent but nonspecific clinical symptoms that denote airflow limitation. Concurrent conditions such as congestive heart failure, pleural effusions, and pulmonary emboli may produce similar symptoms, obscuring the diagnosis.
Diagnostic evaluation
The diagnostic work-up is aimed at establishing a definitive diagnosis, quantifying airflow limitation, and delineating anatomic extent in an effort to optimize therapeutic strategies. The characteristic blunting noted in the flow-volume loop upon pulmonary function testing (PFT) often provides an indication of tracheal obstruction. However, this is a relatively insensitive test, with positive findings noted only with tracheal diameters below 10 mm.6 Spirometry may also precipitate frank respiratory failure in patients with severe airway obstruction and should be used with caution in this subgroup of patients. Rarely, deviation or compression of the trachea may be seen on plain chest radiographs. Plain chest films are otherwise not helpful in defining the anatomic extent of tumor or therapeutic options. Standard chest computed tomography (CT) as well as the latest iterations of low-dose multidetector scanners and advanced airway imaging techniques that allow multiplanar and three-dimensional (3D) reconstruction provides valuable additional information regarding the extent of the lesion and guidance in optimizing therapeutic strategies.7 Bronchoscopy, either flexible or rigid, remains the gold standard in the work-up of airway obstruction. Histologic confirmation of malignancy can be obtained at the time of the examination. Furthermore, bronchoscopy offers direct visualization of the lesion, which permits precise characterization of tumor vascularity and the extent of obstruction, as well as the degree of obstruction attributable to endoluminal versus extraluminal disease. Recent reports have also supported the use of endobronchial ultrasonography as an adjunctive tool in treatment planning.8
Management of malignant airway obstruction
Tumor characteristics, including histologic type, stage, and location, and patient attributes, such as urgency of presentation and performance status, dictate management. Therapeutic strategies vary based on the location and type of obstruction, as well as the local expertise and available institution-specific resources. Surgical resection provides the best prospect for long-term disease control and should be considered in all patients during the initial evaluation. Localized involvement of the small airways and lung parenchyma is best treated by surgical resection, if feasible. In many cases, however, external beam radiotherapy or systemic chemotherapy may be the only treatment options. Patients with central airway obstruction often present with either medically or surgically unresectable disease. While a comprehensive review of the various modalities is beyond the scope of this chapter, some basic principles are outlined (Figure 1). In emergent cases, the barrel of the rigid bronchoscope may be used to mechanically core out the tumor and dilate the airways, providing palliation. Flexible bronchoscopy and balloon bronchoplasty may be used to dilate the airways in less urgent cases.9 Electrocautery, argon plasma coagulation, laser therapy, cryotherapy, brachytherapy, and photodynamic therapy are reasonable approaches for predominantly endoluminal disease (Figure 2). Extraluminal-predominant disease may be best treated with external beam radiotherapy and endobronchial stent placement (Figure 3). Since most lesions are mixed with endo- and extraluminal components, multimodality therapy, using endobronchial laser therapy with mechanical debulking followed by stent placement and subsequent consolidation with external beam radiotherapy, for example, is quite common. Symptom palliation, resulting in reduction in levels of care, may be accomplished in most instances with the judicious application of endoscopic techniques.10 Patients should be evaluated carefully with an early referral to an experienced bronchoscopist who can match the various therapeutic modalities available to the individual patient.
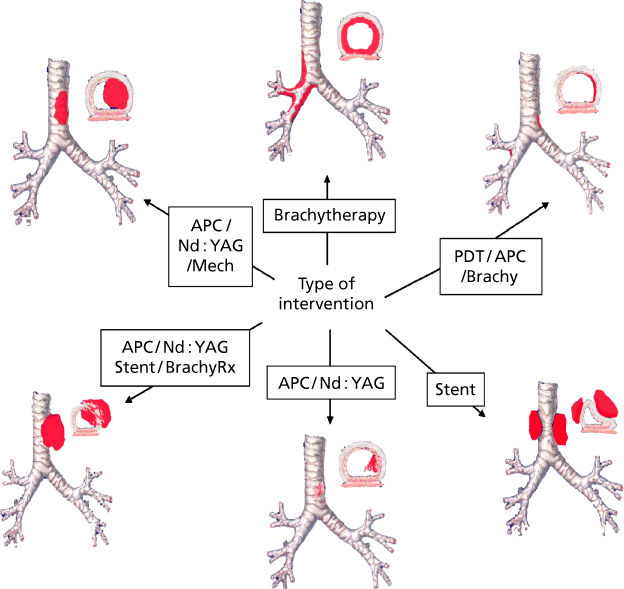
Figure 1 Approach to airway obstruction using interventional bronchoscopic therapy.
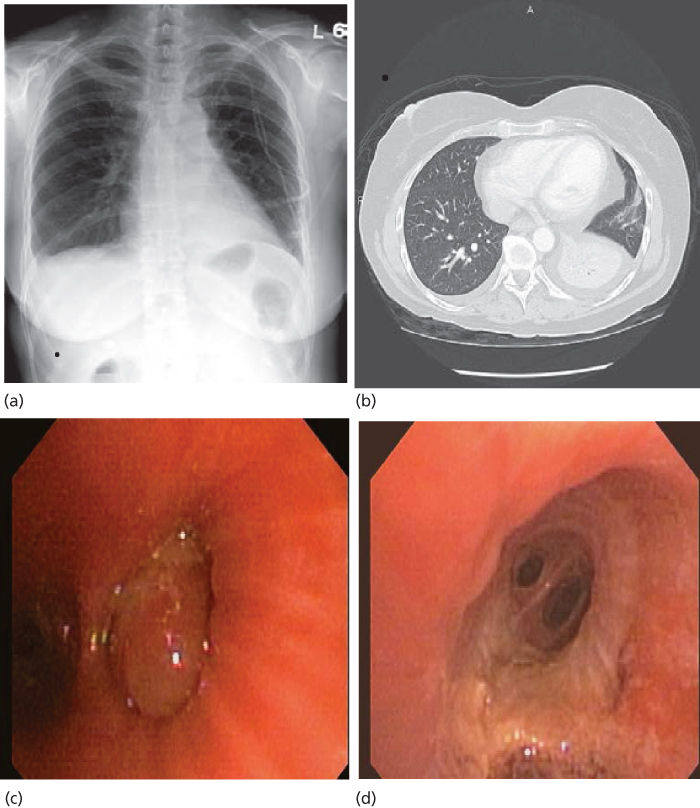
Figure 2 Left lower lobe collapse secondary to metastatic sarcoma (a and b). Complete obstruction of the LLL basilar segments due to a large obstructing tumor was noted at bronchoscopy (c) which was removed using snare forceps and argon plasma coagulation, revealing a patent distal airway (d).
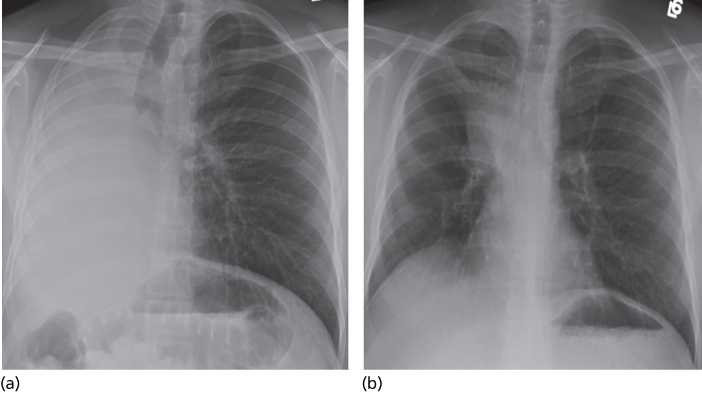
Figure 3 Complete opacification of the right thorax secondary to obstruction of the right mainstem bronchus by a large, predominantly extraluminal mass (a). A wire stent was placed into the bronchus intermedius (b), resulting in partial reexpansion of the right lung.
Malignant pleural effusions
Malignant pleural effusion is a common clinical problem in the cancer setting that signifies distant spread of tumor and, hence, advanced disease. Malignant pleural effusions have significant therapeutic and prognostic implications. Estimates of the incidence of malignant pleural effusions in the United States approach 150,000 cases annually.11 Malignant pleural involvement without effusion occurs in up to 45% of patients with metastatic disease to the pleura.12 In primary pleural malignancies, such as malignant mesothelioma, pleural effusions may be absent. Nearly any type of neoplasm can affect the pleura. Lung cancer accounts for up to half of all malignant effusions, followed in frequency by breast carcinoma and lymphoma. Pleural effusions may occur with acute and chronic leukemias and myelodysplastic syndrome (MDS). Pleural effusions in patients with leukemia are most often due to infection and to a lesser extent leukemic infiltration of the pleura.13 Unfortunately, in 5–10% of patients, a primary tumor cannot be identified.14, 15 Most pleural malignancies arise from tumor emboli to the visceral pleura. The parietal pleura may be involved secondarily, presumably by seeding from the visceral pleura. Direct extensions of tumor from the lung, chest wall, mediastinal structures, or diaphragm and hematogenous metastasis to the parietal pleura are other mechanisms that contribute to the genesis of malignant pleural fluid formation.16 In addition to direct tumor involvement of the pleura, malignant pleural effusions can result from lymphatic blockage anywhere between the parietal pleura and the mediastinal lymph nodes.17 Elevations in the local production of vascular endothelial growth factor (VEGF), a potent mediator of increased vascular permeability, also play a significant role in the formation of malignant pleural effusions.18 Among VEGF homologs, VEGF-D showed a 92.6% rate of positive expression in a study of malignant pleural effusion and may be a useful marker in the diagnosis.19 Seventeen percent of all pleural effusions in patients with cancer are “paramalignant,” a term used for effusions that occur in the setting of cancer that are not caused by direct malignant involvement of the pleural space.16 These effusions develop as a result of local or systemic effects of the tumor, complications of cancer therapy, or concurrent nonmalignant disease.20 Lymphatic obstruction is associated with both malignant and paramalignant effusions and is the most common cause of paramalignant effusions. Other common causes include bronchial obstruction, trapped lung, and pulmonary embolism (PE).
Clinical manifestations, imaging studies, and diagnosis
Most commonly, patients present with symptoms of progressive exertional dyspnea. Cough may also be a troubling symptom with large effusions. Constitutional symptoms are common signals of advanced disease, and thus malaise, weight loss, and poor appetite may become more frequent as the performance status worsens. Hemoptysis and chest wall pain are less common symptoms and may indicate malignant endobronchial disease and tumoral invasion of the chest wall. Standard chest roentgenograms and bilateral decubitus films of the chest provide critical information in the initial evaluation of pleural effusions, including effusion size, position of the mediastinum and diaphragms, presence of loculations and air fluid levels within the pleural space, and characteristics of the underlying lung parenchyma. Knowledge regarding the position of the mediastinum is imperative in therapeutic decision making. Large pleural effusions with contralateral mediastinal shift typically require prompt therapeutic thoracentesis, while those with a centered mediastinum or ipsilateral shift of the mediastinum should be approached cautiously (Figures 4 and 5). In addition to pleural effusions, other disease processes that may cause an ipsilateral shift of the mediastinum or a centered mediastinum with hemithorax opacification include a frozen mediastinum associated with malignant mesothelioma or lymphoma, atelectasis related to occlusion of the ipsilateral central airway, or extensive tumoral infiltration of the ipsilateral lung simulating a large effusion.20 CT is helpful in identifying loculated effusions; offers better anatomical information of the chest wall, parietal and visceral pleura, mediastinal structures, and lung parenchyma; and is especially valuable in delineating alternate diagnoses.21 Ultrasonography provides guidance in locating the optimal site for thoracentesis and is particularly helpful in the setting of loculated pleural effusions. The identification of entrapped lung by ultrasonography using tissue movement may permit deformation (strain) analysis prior to thoracentesis.22, 23 Positron emission tomography (PET) with 18F-fluorodeoxyglucose (FDG) and magnetic resonance imaging (MRI) are both helpful in highlighting extrapleural extension of disease.21 PET imaging provides valuable information associated with malignant mesothelioma; however, its utility in the evaluation of other malignant pleural diseases has not been established. Chemical analysis reveals an exudative effusion in most cases, with only 5% of malignant pleural effusions being transudates.20 Positive pleural fluid cytology, noted in 62% of cases, represents the diagnostic cornerstone of malignant pleural effusions.24 Tumor marker measurements improve the diagnostic yield of cytologically negative effusions by 33% and are particularly valuable when lymphoma, leukemia, or multiple myeloma is suspected.25, 26 The role of flow cytometry in the study of mesotheliomas remains controversial.27 Pleuroscopic pleural biopsies have a 95% sensitivity in the diagnosis of pleural malignancies, and diagnostic yield increases only incrementally (1%) when combined with pleural fluid cytology. By contrast, closed pleural biopsy has a diagnostic yield of only 44% but improves to 77% when combined with an analysis of pleural fluid cytology.24
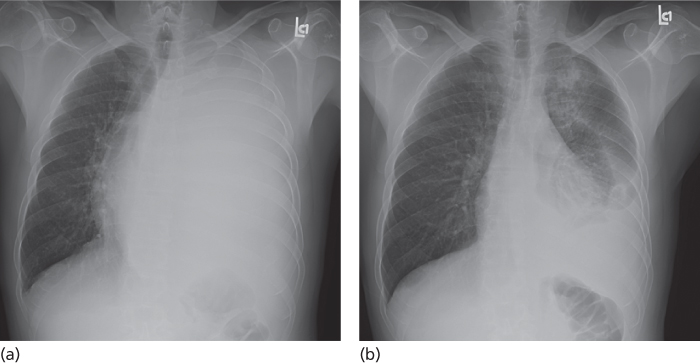
Figure 4 Large left-sided pleural effusion causing opacification of the left hemithorax and contralateral shift of the mediastinum (a). Following thoracentesis (b) the mediastinum shifted back the midline.
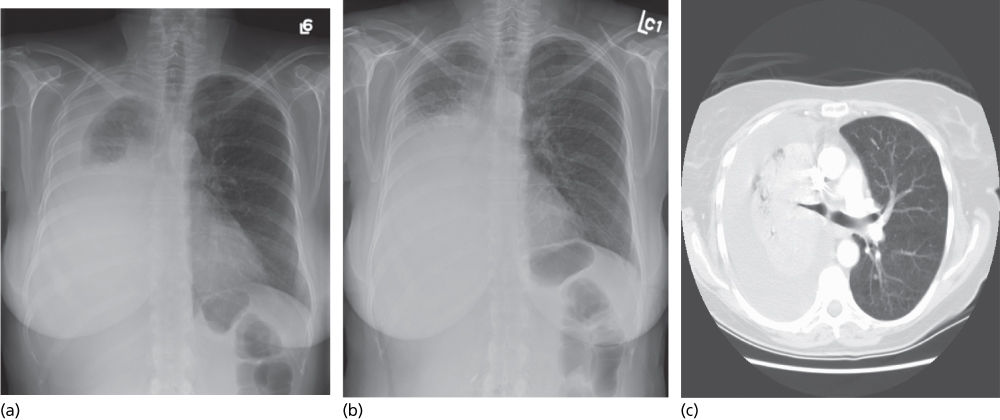
Figure 5 Large right-sided pleural effusion (a) with ipsilateral shift of the mediastinum following thoracentesis (b), indicating volume loss secondary to atelectasis or mass. A CT scan of the chest (C) demonstrates a large mass compressing the right mainstem bronchus.
Management of malignant pleural effusions
Because malignant pleural effusions often signal advanced disease and incurability, treatment efforts are frequently directed toward palliation. Hence, awareness of available therapeutic options tailored to individual patient needs is important. The patient’s performance status and information regarding prior thoracenteses, including the volume of fluid evacuated, whether lung reexpansion and symptom palliation were obtained, and the time interval between repeated taps, are important components of the evaluation that help to guide further therapy. Performance status is the best predictor of survival in patients with recurrent malignant pleural effusions.28 The presence of local chest wall abnormalities, future cancer treatment plans, the patient’s preferences, and the availability of family support influence the approach to these patients. Palliation with simple therapeutic thoracentesis represents a reasonable approach to patients with newly diagnosed chemo- or radiosensitive tumors, such as lymphoma and breast, small-cell lung, germ cell, ovarian, prostate, and thyroid neoplasms, while awaiting response to definitive therapy. After the initial clinical and roentgenographic evaluation, a symptom-limited therapeutic thoracentesis is recommended. A recent consensus statement by the American Thoracic Society and the European Respiratory Society recommends that not more than 1.0–1.5 L of fluid be slowly evacuated from the pleural space in one sitting and that drainage should be discontinued if the patient develops symptoms of dyspnea, cough, or chest discomfort.11 In our experience, patients with radiographic evidence of contralateral mediastinal shift from large pleural effusions may safely tolerate the removal of 2–2.5 L of fluid in one sitting as long as there are no procedure-related symptoms of chest pain, cough, or dyspnea. However, large-volume pleural fluid drainage during a single procedure should be carried out cautiously, especially when radiological studies reveal a centered or ipsilaterally shifted mediastinum. Measurement of pleural pressures during the evacuation of large amounts of fluid may reduce procedure-related complications.29 However, in light of the safety of symptom-limited evacuation, pleural pressure measurement may not be necessary, and its role requires further study. Lung reexpansion following thoracentesis may be assessed with posterior–anterior (PA) and lateral chest radiographs. Repeat thoracenteses spaced 1–2 days apart may be necessary to properly assess lung reexpansion associated with large effusions.30 In 97% of patients, malignant pleural effusions will recur within 1 month, with most of these effusions reappearing within 1–3 days following fluid evacuation.31 Patients with limited life expectancies (<30 days) and poor performance status or those in which pleural fluid reaccumulation is slow are best treated with repeated therapeutic thoracentesis. Repeated thoracentesis is a reasonable approach for patients with malignancies that are expected to respond to chemotherapy and/or radiation therapy (RT). However, frequent thoracentesis may trigger the production of local cytokines and fibrin, resulting in pleural fluid loculation, which not only complicates further thoracenteses but also limits future modes of palliation.32 The use of indwelling pleural catheters has been accepted over the past few years as an alternative palliative option for patients with recurrent malignant effusions. Ideal candidates for this palliative modality include patients with life expectancies in excess of 30 days and in whom prior thoracenteses effected symptomatic relief. Considerations for pleural catheter implantation are valid in this group of patients regardless of lung reexpansion following thoracentesis. Indwelling pleural catheters may be placed in an outpatient setting. Following documentation of proper catheter position, the patient, trained family members, or caregivers may drain the fluid intermittently at home. Daily pleural fluid drainage is recommended initially. During each session, which typically lasts less than 15 min, drainage should be continued until the patient develops cough or chest discomfort, or the fluid stops flowing spontaneously, presumably because the pleural space has been emptied. At our institution, 92% of patients treated with indwelling catheters reported significant relief of dyspnea, and 52% achieved effective pleurodesis. The mean time from catheter insertion to catheter removal was 32 days. Catheter-related complications were observed in only 4% of the patients, including one patient with empyema and two patients with persistent pain at the insertion site. In another study, only 6% of patients required additional drainage of fluid following removal of the catheter.33 Patients who have chemotherapy or radiation after catheter placement and those who are more short of breath at baseline have greatest improvements in utility.34 In a subgroup analysis, patients meeting criteria for a pleurodesis procedure achieved 70% effective pleurodesis after insertion of indwelling pleural catheter.35
Traditionally, chemical pleurodesis has been the most widely used method to control recurrent malignant pleural effusions. Unfortunately, the lack of prospective studies precludes comparative analyses of efficacy, safety, and cost of the existing chemical agents and pleurodesis techniques. The results from available literature suggest that nonchemotherapeutic agents are more efficacious and the most cost-effective sclerosants.11 Sterilized, asbestos-free talc is the preferred pleurodesing agent. Complications vary with the surface characteristics of the talc particles. The use of talc particles that are less than 5 mm in size has been associated with pulmonary injury, including acute pneumonitis and respiratory failure associated with adult respiratory distress syndrome, and should be avoided.36 The safety of large-particle talc for pleurodesis was recently confirmed in a European multicenter trial in which no association with adult respiratory distress syndrome was identified.37 The superiority of thoracoscopic talc insufflation over talc slurry as a method of administration of the sclerosant is a matter of debate. Success rates of >90% have been reported for both techniques, without significant differences in the rate of overall complications or disease recurrence.11 The largest published prospective randomized trial comparing thoracoscopic talc insufflation and talc slurry included 501 patients with a performance status of 1 or 2. Results showed no difference in 30-day survival rates and pleurodesis success rates between chest tube talc slurry and thoracoscopic talc poudrage. Unexpected high morbidity and mortality rates were reported in both groups. A subset analysis suggested that thoracoscopic talc insufflation may be advantageous for patients with lung or breast cancer.38 Based on the available information, our group considers all patients with a good performance status (ECOG 0, 1, or 2), and in whom symptomatic relief and lung reexpansion was achieved after initial drainage of the pleural fluid, for either indwelling pleural catheter placement or pleurodesis with pleuroscopic talc poudrage as the preferred palliative modalities. Rarely, alternative modalities such as pleuroperitoneal shunts and parietal pleurectomy are used in the management of recurrent symptomatic effusions following pleurodesis failures or effusions associated with trapped lung. Chylous effusions associated with malignancy are controlled by treating the primary tumor. Prolonged loss of chyle, a protein-rich, fat-laden, and lymphocyte-predominant fluid, may result in lymphopenia, severe nutritional depletion, and water and electrolyte loss. Mortality due to chylothorax can be as high as 50%. Among those patients with recurrent symptomatic chylothorax and cancer relapse or progressive disease despite adequate treatment, parenteral alimentation and talc pleurodesis39 and indwelling pleural catheter placement represent reasonable treatment alternatives.40 Pleuroperitoneal shunt placement is an attractive option, since chyle is not lost but mobilized from the thorax to the peritoneum where it is reabsorbed, thus mitigating the risk of malnourishment and immune suppression. Unfortunately, the pleuroperitoneal shunt pump mechanism displaces only 1.5–2.5 mL at a time, making its use cumbersome. In addition, the incidence of obstruction of the pump is high. Peritoneal tumor seeding through the shunt is a theoretical concern, although this has not been clearly documented. Embolization of the thoracic duct represents an alternative strategy in the management of recurrent chylous effusions. This procedure appears to be well tolerated, but definitive evidence of its efficacy in the cancer population is not available. Parietal pleurectomy, decortication, and pleuropneumonectomy are associated with high mortality rates and do not provide better symptom control than other palliative options.11 The utilization of compounds to block VEGF either alone or in combination with other palliative modalities is promising. Theoretically, decreasing production of pleural fluid with VEGF blockade followed by drainage of the pleural effusion and instillation of a chemical agent to obtain pleural symphysis could accelerate pleurodesis and reduce hospital stay.41 Regrettably, the final results of a single-arm phase II clinical trial of the VEGF receptor inhibitor, vandetanib, combined with intrapleural catheter placement in patients with non-small-cell lung cancer (NSCLC) and recurrent malignant pleural effusion did not significantly reduce time to pleurodesis.42
In summary, in patients with limited life expectancies, modalities that offer the best chance for palliation of symptoms, the lowest procedure-related morbidity and mortality, and the shortest hospital stay represent a reasonable approach to the management of recurrent malignant effusions. A multidisciplinary approach (Figure 6), involving oncology, pulmonary medicine, interventional radiology, and thoracic surgery, offers the best opportunities to achieve these goals.
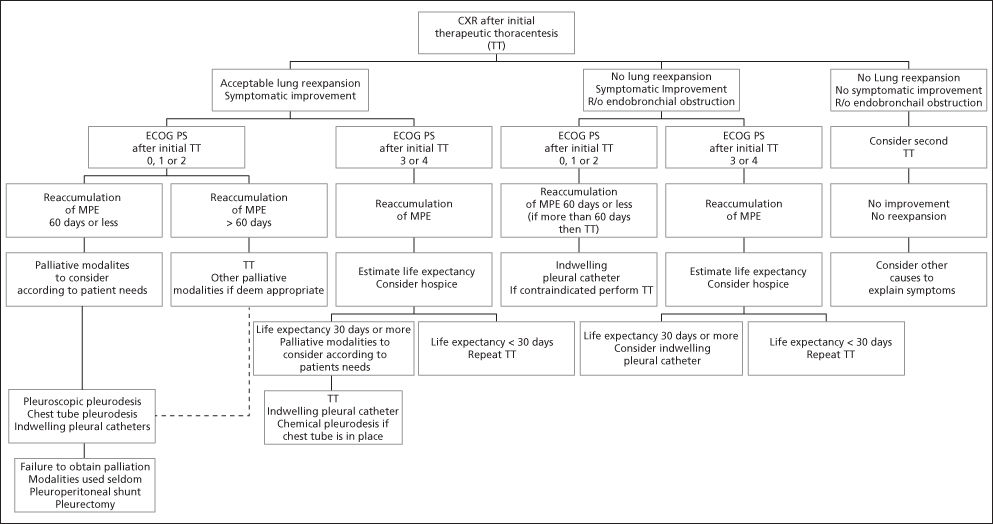
Figure 6 Management of malignant pleural effusions (MPE). Patients with chemo- or radiosensitive tumors on initial treatment (lymphoma, breast cancer, small-cell lung cancer, germ cell cancer, ovarian cancer, prostate cancer, and thyroid neoplams) may be candidates for therapeutic thoracentesis while awaiting systemic treatment results.
Postsurgical respiratory insufficiency
Diagnostic evaluation
The initial approach to the patient with an anatomically resectable tumor includes strategies to determine the patient’s functional operability and the predicted long-term pulmonary disability following the loss of the resected lung. This may be accomplished through pulmonary-specific testing as well as a general assessment aimed at identifying and optimizing control of any coexisting systemic diseases. The pulmonary evaluation consists of three sequential steps: (1) measurement of baseline pulmonary function, (2) quantitative radionuclide regional ventilation/perfusion (V/Q) pulmonary studies to calculate postoperative lung function, and (3) exercise testing for patients that do not meet acceptable results on the two previous steps.43 Among the PFTs that have been used as predictors of postoperative outcome, reduced values of forced expiratory volume in 1 second (FEV1) and diffusing capacity for carbon monoxide (DLCO) are the most reproducible and most frequently used for predicting complications of lung resection.44 For decision making, values of FEV1 reported as percent of predicted that take into account variations in patients’ height, gender, and race are preferred over values reported in absolute units (L). In our laboratory, more than half of patients with an FEV1 between 60% and 80% of predicted have an estimated postpneumonectomy FEV1 by radionuclide studies that is below acceptable values for safe resection (<40% of predicted). Therefore, we recommend that only those patients with baseline FEV1 and DLCO ≥ 80% of predicted and no clinical evidence of contralateral pulmonary disease be considered for resection without further testing. All other patients should undergo a “split function” evaluation, which is a quantitative radionuclide assessment of regional lung ventilation and/or perfusion. In this study, the uptake of radioactive ions by various regions in each lung is measured by inhalation of 133Xe or by intravenous administration of 133Xe dissolved in saline or 99Tc macroaggregates. In practice, estimates of lung perfusion alone are easiest and most commonly measured. The percentage of radioactivity contributed by each lung correlates with the contribution to the overall function by that lung. The predicted postoperative FEV1 (FEV1ppo) and predicted postoperative DLCO (DLCOppo) are calculated by subtracting the percent functional uptake of the region to be resected from the total uptake. Several investigators have documented the usefulness of split function studies for predicting both the risk of complications and the loss of pulmonary function after pulmonary resection.45, 46 In these studies, preoperative predicted values are close to measured postoperative values for pneumonectomy and for resections involving more than three segments.47 Pulmonary function remains relatively stable after pneumonectomy. Predictions for a smaller resection, such as a lobectomy, however, are less reliable, owing to a disproportionate early loss, followed by significant functional improvement with time.48 Kearney et al.49 also described a low FEV1ppo as the only significant independent predictor of complications. Other variables, including age ≥ 60, male sex, history of smoking, pneumonectomy, hypercarbia, (pCO2 ≥ 45 mm Hg), desaturations on exercise oximetry (SaO2 ≤ 90%), and a preoperative FEV1 ≤ 1 L were not predictive of complications. Markos et al.50 reported that a DLCOppo <40% of predicted was associated with higher morbidity and mortality and was the best predictor of postoperative respiratory failure. In summary, an FEV1ppo and DLCOppo of ≥ 40% of predicted on split function studies represent safe preoperative criteria for lung resection, including pneumonectomy. Patients that do not meet these criteria but are candidates for lesser surgeries, such as a lobectomy or segmentectomy, should undergo further evaluation with exercise testing.
The rationale for using exercise testing in these high-risk patients is based on two concepts: (1) lung function is not the only determinant of performance, and (2) losses for lobectomies or lesser resections improve over time and tend to be overestimated by radiospirometric studies.43 Exercise testing also offers the advantage of examining cardiopulmonary and musculoskeletal interactions during stress in a single study. The most validated form of exercise testing is cycle ergometry with incremental workloads to the symptom-limited maximum (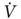 O2peak). Using this method, Smith et al.51 found that only 1 of 10 patients with a
O2peak). Using this method, Smith et al.51 found that only 1 of 10 patients with a 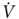 O2peak > 20 mL/kg/min developed complications postoperatively, whereas all patients with a
O2peak > 20 mL/kg/min developed complications postoperatively, whereas all patients with a 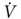 O2peak < 15 mL/kg/min had complications. We conducted two studies on patients that had been considered inoperable because of FEV1 ≤ 40% of predicted, FEV1ppo ≤ 33% of predicted, and/or arterial PCO2 ≥ 45 mm Hg. Patients that reached a
O2peak < 15 mL/kg/min had complications. We conducted two studies on patients that had been considered inoperable because of FEV1 ≤ 40% of predicted, FEV1ppo ≤ 33% of predicted, and/or arterial PCO2 ≥ 45 mm Hg. Patients that reached a 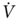 O2peak ≥15 ml/kg/min underwent surgical treatment; others were referred to radiation and/or chemotherapy. All surgically treated patients were extubated within 24 h, and the median time to discharge following surgery was 8 days. There were no inhospital deaths, although reversible postoperative complications occurred in 40% of the patients. Moreover, a survival benefit among these high-risk patients treated surgically was noted.52, 53 More recently, we determined that values of
O2peak ≥15 ml/kg/min underwent surgical treatment; others were referred to radiation and/or chemotherapy. All surgically treated patients were extubated within 24 h, and the median time to discharge following surgery was 8 days. There were no inhospital deaths, although reversible postoperative complications occurred in 40% of the patients. Moreover, a survival benefit among these high-risk patients treated surgically was noted.52, 53 More recently, we determined that values of 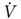 O2peak expressed as percent of predicted more accurately estimated surgical risk and helped to maximize the number of patients that can safely undergo lung resection. We concluded that high-risk patients that achieve a
O2peak expressed as percent of predicted more accurately estimated surgical risk and helped to maximize the number of patients that can safely undergo lung resection. We concluded that high-risk patients that achieve a 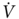 O2peak ≥ 60% of predicted during exercise have an acceptable outcome after lung resection, even if
O2peak ≥ 60% of predicted during exercise have an acceptable outcome after lung resection, even if 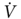 O2peak < 15 mL/kg/min.33 Our approach to preoperative assessment for lung resection is summarized in Figure 7. In addition to an estimation of surgical risk and postoperative function, the goals of preoperative assessment include the development of strategies to reduce the risk and maximize the number of patients that can benefit from surgical therapy. Finally, one must keep in mind that there is no test that will predict all complications and that the patient and the surgeon should make the final decision regarding the risk/benefit of surgical treatment.
O2peak < 15 mL/kg/min.33 Our approach to preoperative assessment for lung resection is summarized in Figure 7. In addition to an estimation of surgical risk and postoperative function, the goals of preoperative assessment include the development of strategies to reduce the risk and maximize the number of patients that can benefit from surgical therapy. Finally, one must keep in mind that there is no test that will predict all complications and that the patient and the surgeon should make the final decision regarding the risk/benefit of surgical treatment.
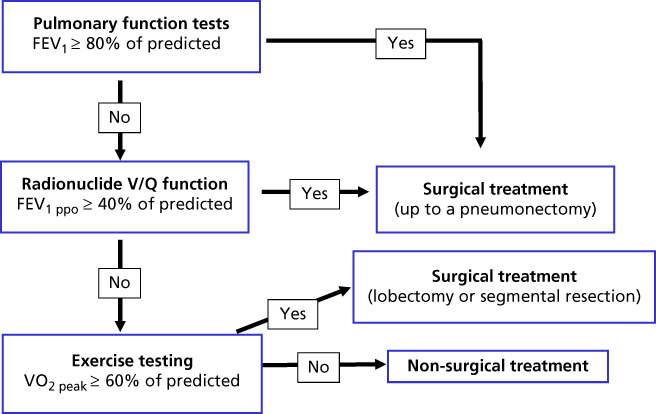
Figure 7 Approach to preoperative evaluation for lung resection.
Chemotherapy-induced lung injury
Conventional chemotherapy, as well as a growing list of molecularly targeted antineoplastic agents, is frequently implicated in lung injury. Lung toxicity in this setting may be idiosyncratic, unpredictable, and highly variable from one therapeutic class of drugs to the next. In addition, individual agents within a certain therapeutic class may induce similar patterns of lung injury with widely varying frequencies. For example, among the class of small-molecule tyrosine kinase inhibitors (TKIs) that target the epidermal growth factor receptor (EGFR), gefitinib and erlotinib have been associated with sometimes fatal interstitial pneumonitis (IP), while lung toxicity following use of the dual EGFR-HER2 TKI lapatinib is very rare.54 Similarly, fatal lung injury following administration of the cytotoxic alkylating agents busulfan and cyclophosphamide is well recognized, whereas lung injury from the alkylating agent chlorambucil is very unusual and has been confined to isolated case reports.55–57
Lung injury associated with conventional chemotherapy
The pathogenesis of chemotherapy-related lung injury is poorly understood. Proposed mechanisms include direct cytotoxicity to type II pneumocytes and/or the alveolocapillary endothelium, cytokine release resulting in endothelial dysfunction and capillary leak syndrome, oxidative injury associated with the release of free oxygen radicals, and/or cell-mediated lung mechanisms.58–60
Individual drug toxicities may be confined to the pulmonary interstitium, alveoli, pleura, pulmonary circulation, or airways, or, alternatively, may involve multiple intrathoracic structures. The response of the lung to drug-induced injury (DLI) is very limited, resulting in stereotyped histopathologic injury patterns or syndromes. Predominant interstitial and alveolar injury patterns are most common and include various patterns of IP, such as nonspecific interstitial pneumonitis (NSIP), desquamative interstitial pneumonia (DIP), hypersensitivity pneumonitis (HP), organizing pneumonia (OP), and eosinophilic pneumonia (EP). Noncardiogenic pulmonary edema (NCPE), diffuse alveolar damage (DAD), acute respiratory distress syndrome (ARDS), and diffuse alveolar hemorrhage (DAH) are other forms of DLI-related injury patterns. In addition to direct lung injury, chemotherapy-induced immune suppression may predispose patients to life-threatening infections, of which pneumonia is most common. Drug-induced granulomatous disease and lymphadenopathy have also been described. These lung injury patterns have been reported following conventional chemotherapeutic regimens as well as molecular targeted and immune modulator therapies. Patterns of lung injury associated with conventional and molecularly targeted cancer therapies are shown in Tables 1 and 2, respectively.
Table 1 Major clinical syndromes and histologic patterns of chemotherapy-induced lung injury
| Agent class | Specific agent | DAD, | Hemoptysis | NCPE | IP | EP | Granuloma | Bronchospasm/IR | BOOP | PHTN | VTE/PE | Pleural | Opportunistic | MetHgb | Radiation |
| ARDS, | (unrelated | formation | (PVOD, | disease | infections | recall | |||||||||
| DAH | to DAH) | PAH) | pneumonitis | ||||||||||||
| Alkylating agents | Busulfan | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Cyclophosphamide | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Ifosfamide | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Temozolomide | ✓ | ✓ | ✓ | ||||||||||||
| Oxaliplatin | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Carboplatin | ✓ | ||||||||||||||
| Cisplatin | ✓ | ||||||||||||||
| Melphalan | ✓ | ✓ | |||||||||||||
| Antimetabolites | Methotrexate | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Azathioprine | ✓ | ✓ | ✓ | ||||||||||||
| Cytarabine | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Fludarabine | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Azacitabine | ✓ | ||||||||||||||
| Gemcitabine | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Pentostatin | ✓ | ✓ | |||||||||||||
| Pemetrexed | ✓ | ✓ | |||||||||||||
| Zinostatin | ✓ | ✓ | |||||||||||||
| Cytotoxic antibiotics | Bleomycin | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Mitomycin C | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Amrubicin | ✓ | ✓ | |||||||||||||
| Liposomal doxorubicin | ✓ | ✓ | ✓ | ||||||||||||
| Topoisomerase inhibitors | Irinotecan | ✓ | |||||||||||||
| Topotecan | ✓ | ✓ | ✓ | ||||||||||||
| Podophyllotoxins | Etoposide | ✓ | ✓ | ✓ | |||||||||||
| Teniposide | ✓ | ||||||||||||||
| Nitrosoureas | BCNU | ✓ | ✓ | ✓ | ✓ | ||||||||||
| CCNU | ✓ | ||||||||||||||
| Taxanes, microtubule inhibitors | Paclitaxel | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||
| Docetaxel | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Vincristine | ✓ | ✓ | |||||||||||||
| Vinblastine | ✓ | ✓ | |||||||||||||
| Vindesine | ✓ | ✓ | |||||||||||||
| Vinorelbine | ✓ | ✓ | ✓ | ||||||||||||
| Ixabepilone | |||||||||||||||
| Other | All-trans-retinoic acid | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Arsenic trioxide | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Procarbazine | ✓ | ✓ | ✓ | ✓ | |||||||||||
| L-asparaginase | ✓ | ✓ |
DAD, diffuse alveolar damage; ARDS, acute respiratory distress syndrome; DAH, diffuse alveolar hemorrhage; NCPE, noncardiogenic pulmonary edema; IP, interstitial pneumonitis; EP, eosinophilic pneumonia; IR, infusion reaction; BOOP, bronchiolitis obliterans with organizing pneumonia; PHTN, pulmonary hypertension: PVOD, pulmonary venoocclusive disease; PAH, pulmonary arterial hypertension; VTE/PE, venous pulmonary embolism/pulmonary embolism; MetHgb, methemoglobinemia.
Table 2 Major clinical syndromes and histologic patterns of lung injury associated with molecularly targeted agents
| Agent class | Specific agent | DAD, | Hemoptysis | NCPE | IP | EP | Granuloma | Bronchospasm | BOOP | PHTN | VTE/PE | Pleural | Opportunistic | MetHgb | Radiation |
| ARDS, | (unrelated | formation | (IR/CR) | (PVOD, | disease | infections | recall | ||||||||
| DAH | to DAH) | PAH) | pneumonitis | ||||||||||||
| Monoclonal antibodies | Cetuximab | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Panitumumab | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Bevacizumab | ✓ | ✓ | ✓ | ||||||||||||
| Alemtuzumab | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Rituximab | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Obinutuzumab | ✓ | ||||||||||||||
| Ofatumumab | ✓ | ✓ | ✓ | ||||||||||||
| Ibritumomab | ✓ | ✓ | ✓ | ||||||||||||
| Trastuzumab | ✓ | ||||||||||||||
| Pertuzumab | ✓ | ✓ | |||||||||||||
| Gemtuzumab | ✓ | ✓ | |||||||||||||
| Ipilimumab | ✓ | ✓ | |||||||||||||
| Tyrosine kinase inhibitors | Gefitinib | ✓ | ✓ | ||||||||||||
| Erlotinib | ✓ | ✓ | ✓ | ||||||||||||
| Imatinib | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Dasatinib | ✓ | ✓ | ✓ | ||||||||||||
| Bosutinib | ✓ | ||||||||||||||
| Ponatinib | ✓ | ||||||||||||||
| Sorafenib | ✓ | ✓ | ✓ | ✓ | |||||||||||
| Sunitinib | ✓ | ✓ | |||||||||||||
| Pazopanib | ✓ | ✓ | |||||||||||||
| Vandetanib | ✓ | ✓ | |||||||||||||
| Idelalisib | ✓ | ✓ | |||||||||||||
| Trametinib | ✓ | ||||||||||||||
| Crizotinib | ✓ | ✓ | ✓ | ||||||||||||
| Vemurafenib | ✓ | ||||||||||||||
| Ruxolitinib | ✓ | ✓ | |||||||||||||
| Rapamycin inhibitors | Everolimus | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Temsirolimus | ✓ | ✓ | |||||||||||||
| Proteasome inhibitors | Bortezomib | ✓ | ✓ | ✓ | ✓ | ||||||||||
| Carfilzomib | ✓ | ✓ | |||||||||||||
| Immune modulators | Thalidomide | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||||
| Lenalidomide | ✓ | ||||||||||||||
| Pomalidmine | |||||||||||||||
| IL-2 | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||||||
| TNF | ✓ | ||||||||||||||
| IFN-g | ✓ | ✓ | ✓ | ✓ | ✓ |
DAD, diffuse alveolar damage; ARDS, acute respiratory distress syndrome; DAH, diffuse alveolar hemorrhage; NCPE, noncardiogenic pulmonary edema; IP, interstitial pneumonitis; EP, eosinophilic pneumonia; IR, infusion reaction; BOOP, bronchiolitis obliterans with organizing pneumonia; PHTN, pulmonary hypertension: PVOD, pulmonary veno-occlusive disease; PAH, pulmonary arterial hypertension; VTE/PE, venous pulmonary embolism/pulmonary embolism; MetHgb, methemoglobinemia.
Estimates of the incidence of DLI caused by individual agents are hampered by the frequent use of complex multidrug and multimodality regimens given either concomitantly or sequentially. Predisposing factors such as older age, cumulative dose, concomitant or sequential radiotherapy, oxygen administration, prior lung injury, and the use of multidrug regimens significantly influence both the occurrence and latency periods between drug exposure and the development of clinical symptoms.
Overlapping clinical, radiographic, and pathologic manifestations of lung injury caused by cardiogenic and NCPE, infections, cancer relapse, or RT confound clinical distinctions between these entities and also render precise estimates of DLI difficult. Other conditions that may mimic DLI include aspiration pneumonitis, cardiogenic pulmonary edema, acute lung injury (ALI), and ARDS.61, 62 Clinical symptoms range from low-grade fever with dry cough and dyspnea to fulminant disease which may rapidly progress to respiratory failure and ARDS. Skin rash, flushing, and bronchospasm, when present, may suggest either an allergic hypersensitivity reaction or a non-IgE-mediated pseudoallergic reaction. These symptoms may occur within minutes to hours of therapy. In a recent study, direct activation of mast cells by cationic drugs was found to be the cause of pseudoallergic reactions.63 Allergic reactions typically require a sensitization period, with symptom onset during the second or subsequent cycles of therapy. Pseudoallergic reactions, by contrast, may occur within minutes after administration of the first cycle of chemotherapy. Clinical manifestations of other forms of DLI, including IP, typically occur early, within weeks to a few months after initiation of therapy.59, 60 Delayed pulmonary fibrosis, occurring months to years after exposure to bleomycin, busulfan, cyclophosphamide, gemcitabine, and the nitrosoureas as a late manifestation of DLI, has also been described. 64–67
Systemic markers of inflammation, such as leukocytosis, elevated erythrocyte sedimentation rate, and C-reactive protein, are common but nonspecific findings. Derangements on PET are nonspecific but nonetheless important in assessing the degree of DLI-related pulmonary impairment. Reductions in the DLCO are generally accepted as the most sensitive parameter in the assessment of DLI, although the predictive potential for the detection of early change has been variable.68–73 With disease progression, a restrictive ventilatory defect may be seen.65, 74 Near-complete normalization of pulmonary function within 2 years of exposure is common following some forms of chemotherapy-induced lung injury.73 Imaging studies, including high-resolution computed tomography (HRCT) scans, are important tools in the evaluation of patients with suspected DLI, although the findings are nonspecific. Interstitial and mixed alveolar–interstitial abnormalities, manifested as ground-glass opacities that localize to the peripheral and lower lung zones, are the most frequent radiographic findings on CT. Upper lung zone predominant infiltrates are also seen, particularly following drug-induced hypersensitivity reactions. Nodular lesions may mimic underlying malignancy. Reticular lines, septal thickening, and mosaic attenuation are also observed. Pulmonary fibrosis associated with traction bronchiectasis and honeycomb patterns may be seen as the disease progresses. Diffuse uptake of 18F-FDG on PET–CT imaging has been observed in patients with radiographic changes suggestive of pneumonitis following bleomycin-, etoposide-, and rituximab-based therapies. Similar findings may also be seen with lymphangitic spread of tumor.75, 76
Bronchoscopy with performance of bronchoalveolar lavage (BAL) may be helpful in excluding competing diagnoses of infection or background disease. Studies have shown diagnostic yields as high as 70–90% for pulmonary infections and 35–70% for lymphoma and lymphangitic spread of lung cancers.77, 78 BAL fluid in DLI is typically hypercellular with increased numbers of neutrophils or lymphocytes. Decreased CD4/CD8 ratios on BAL fluid are supportive findings; however, ratios vary widely and cannot distinguish sufficiently between drug-induced versus other causes of interstitial lung diseases (ILDs).79 Drug-induced HP is suggested by BAL lymphocytosis of greater than 50%, with a low CD4 to CD8 ratio. BAL eosinophilia of greater than 25% is supportive of drug-induced EP. The presence of DAH is supported by progressively bloody BAL samples on sequential saline aliquots and/or cytologic evidence of increased numbers of hemosiderin-laden macrophages on BAL fluid. Transbronchial and surgical lung biopsies may be helpful in documenting IP, DAD, EP, and OP and excluding competing diagnoses such as vasculitis, infection, and underlying malignancy. Although pathologic findings attributable to DLI may be seen in 20% of tissue biopsies, histopathologic criteria for DLI have not been established, and no findings are considered pathognomonic. Nonetheless, lung biopsies are useful in excluding competing diagnoses and characterizing the histopathologic pattern of lung injury. This information is not only useful in diagnosing DLI but also in guiding therapeutic options. The diagnosis of DLI is usually based on the temporal association between drug exposure and the development of pulmonary injury; the integration of compatible clinical, radiographic, and laboratory findings; and the exclusion of competing diagnoses.
Treatment of chemotherapy-related DLI remains anecdotal and empiric, rather than evidence based. Withdrawal of the suspected offending agent is the mainstay of treatment in the majority of cases. Spontaneous improvement may be seen in most patients following drug withdrawal, although disease progression may occur even after withdrawal of the offending agent. General recommendations suggest discontinuation of the culprit drug once sufficient clinical suspicion to support its association with pneumotoxicity has been established. One clear exception to this recommendation has been in the setting of the differentiation syndrome following all-trans-retinoic acid (ATRA) or arsenic trioxide therapies for treatment of acute promyelocytic leukemia.80 Systemic steroid therapy along with de-escalation of drug dose rather than drug withdrawal has been associated with successful resolution of toxicity in patients with mild to moderate forms of this syndrome.
Systemic glucocorticoids and supportive care (including inhaled bronchodilators, supplemental oxygen, and mechanical ventilation) should be initiated if clinically indicated. Corticosteroids have been shown to abrogate symptoms of DLI in certain steroid-responsive lung injury patterns, such as HP, EP, and bronchiolitis obliterans with organizing pneumonia (BOOP). In other entities, including pulmonary fibrosis, pulmonary vascular disease, and bronchiolitis obliterans (BO), corticosteroids have no beneficial role. Current expert opinion advocates close surveillance with high-resolution CT scans and continued therapy without dose interruption for asymptomatic ILD. For symptomatic patients, the grade of pneumotoxicity should be used to guide management decisions, which may include dose modification or interruption, with or without the institution of corticosteroid therapy.81 For patients with moderate (grade 2 or greater) IP, drug interruption and initiation of corticosteroid therapy, dosed at 0.75–1 mg/kg/day of prednisone or its equivalent, are recommended. Steroids are typically maintained at the higher dose until symptom improvement is established and then tapered over a 1–3-month time period, pending the response to therapy. Bronchoscopy with alveolar lavage is recommended to rule out competing diagnoses of infection and/or alveolar hemorrhage.82 Drug rechallenge is generally not recommended, except in specific settings, such as ATRA and arsenic-related differentiation syndrome.80, 83
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree