INTRODUCTION
Breast cancer is one of the most commonly diagnosed malignancies in women of childbearing age. Approximately 10% of women diagnosed with breast cancer are younger than 45, translating to over 23,000 women in the United States yearly and many thousands more worldwide (
1). Thanks to improvements in the treatment of breast cancer and an increasing focus on survivorship issues, combined with the sociodemographic trend of delaying childbearing, many young breast cancer survivors are interested in future fertility (
2,
3 and
4). A recent metaanalysis found that concerns about infertility contributed to high levels of distress in young women with breast cancer (
5). Breast cancer treatment can diminish or destroy a woman’s reproductive potential due to direct gonadal toxicity or due to the natural waning of fertility while she is receiving therapy. However, many young women remain premenopausal and fertile for at least some period of time after breast cancer treatment, and some are interested in interventions to increase the chance that they will be able to conceive (
6).
Because of the intricate relationship between breast cancer and hormones, fertility and reproductive issues in this population are complex. Reproductive factors are associated with the risk of developing breast cancer, and hormonal manipulations and medications are a mainstay of breast cancer treatment. Chemotherapy recipients who experience treatment-related amenorrhea have a better prognosis than women who continue to menstruate throughout chemotherapy (
7). Nevertheless, for some young women with breast cancer, the threat or experience of infertility may be particularly distressing (
6,
8). Fertile Hope is an advocacy organization that supports fertility preservation for cancer patients; its founder proclaimed about her cancer diagnosis on
www.fertilehope. org, “The thought of being sterile was almost as devastating as my cancer diagnosis itself.” Some women are also interested in avoiding potential ill-health effects of premature menopause, including hot flashes, vaginal dryness, bone thinning, cardiovascular problems, and mental health issues (
9,
10).
EFFECT OF BREAST CANCER TREATMENT ON OVARIAN FUNCTION
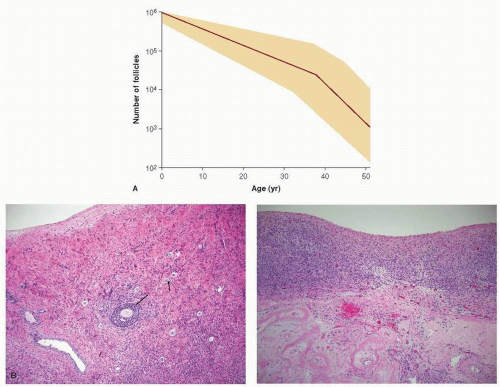
Fertility may be adversely affected in several ways in young women with breast cancer. First, the time required to receive systemic breast cancer treatment may be months (e.g., conventional cytoxic chemotherapy) or years (e.g., adjuvant tamoxifen). During treatment, while pregnancy is contraindicated due to risks of teratogenicity, ovarian function and fertility are naturally declining due to aging. (see
Fig. 90-1). The average age of menopause in the United States is 51 years, but fertility wanes many years before cessation of menses for most women, so many breast cancer patients diagnosed in their late 30s or 40s will be unable to conceive by the time they complete five to ten years of treatment with tamoxifen.
Second, adjuvant chemotherapy is directly toxic to the ovary, reducing fertility and potentially inducing premature menopause. The degree of damage to the ovaries will determine whether amenorrhea is temporary or permanent. Chemotherapy induces apoptosis in dividing cells in the ovary including maturing follicles (
11). Because alkylating agents like cyclophosphamide are not cell-cycle specific, they may also directly kill oocytes and pregranulosa cells of primordial follicles. There is mixed evidence regarding whether chemotherapy given during the follicular phase of the menstrual cycle is more injurious to ovarian function than chemotherapy given at other times (
12,
13). Chemotherapy-associated premature menopause is thought to occur in 10% to 90% of patients receiving chemotherapy depending on the regimen given, the age of the patients, and the definition of menopause. Most studies are limited by the use of chemotherapy-related amenorrhea (CRA) as a
surrogate for menopause and infertility. Treatment regimens vary substantially, and follow-up is heterogeneous and relatively short. CRA may be temporary, especially in very young women; older women are more likely to have permanent amenorrhea. Chronically anovulatory women may remain fertile even if they are not having menstrual cycles, but, on the other hand, ongoing menses are a poor surrogate for fertility, especially as women age, due to waning egg quality.
Available data confirm that risk of CRA is related to increasing age and increasing cumulative dose of cytotoxic chemotherapy, in particular, alkylating agents (
14). A recent study of more than 2,000 pre- and perimenopausal women randomized to docetaxel-doxorubicin versus concurrent docetaxeldoxorubicin-cyclophosphamide or sequential doxorubicincyclophosphamide followed by docetaxel found that the women in the non-cyclophosphamide containing group had significantly less likelihood of CRA (
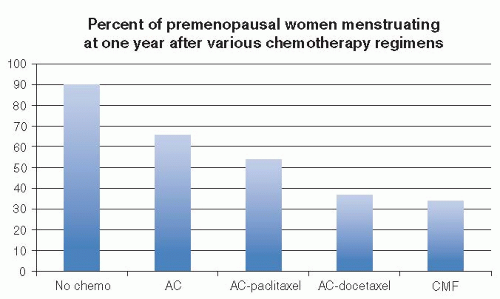
15). Younger age was also significantly protective against CRA. Likewise, an earlier prospective longitudinal survey of 595 U.S. women with breast cancer diagnosed at age 25 to 40 undergoing adjuvant chemotherapy confirmed that menstrual cycles were less likely to persist at 1 year among women treated with regimens containing higher cumulative doses of cyclophosphamide (i.e., cyclophosphamide-methotrexate-5-fluorouracil [CMF] or 5-fluorouracil-doxorubicin-cyclophosphamide rather than doxorubicin-cyclophosphamide, doxorubicin-cyclophosphamide-paclitaxel, or doxorubicin-cyclophosphamidedocetaxel) (OR 0.37, 95% CI, 0.37-0.67) (
16) (see
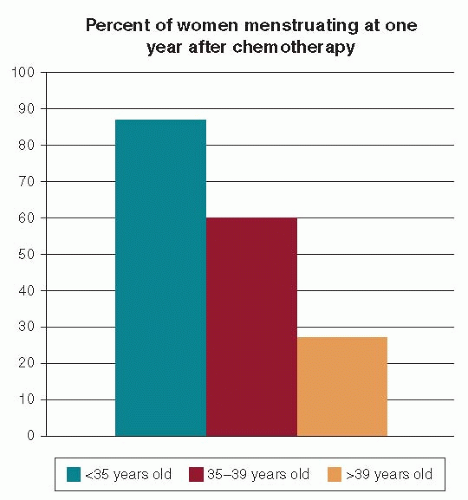
Fig. 90-2). Rates of menstrual bleeding six months after completion of chemotherapy were also strongly related to patient age, with approximately 85% of women age <35 years having ongoing menses, 61% in women ages 35 to 40, and <25% in those age >40 (see
Fig. 90-3). Recent evidence suggests that the addition of the taxanes to anthracycline-based adjuvant chemotherapy confers little or no increased risk of CRA, although data are mixed (
17,
18,
19 and
20).
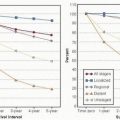
Table 90-1 summarizes the risk of CRA with common adjuvant therapy regimens by age (
14).
Even among women who remain premenopausal after cytotoxic therapy, menopause may ensue earlier than would have been expected in the absence of chemotherapy. An analysis of
the International Breast Cancer Study Group (IBCSG) Trials V and VI revealed that 227 women who were menstruating and disease free at 2 years after diagnosis and treatment with 6 to 7 cycles of CMF had earlier menopause compared to controls (
21). For a woman who was 30 years old at time of diagnosis and menstruating 24 months after 6 cycles of CMF, there was a 37% risk of menopause only 3 years later (at age 35) and an 84% risk at age 40. Other studies have also shown that ovarian reserve is diminished even in young women who remain premenopausal after chemotherapy for breast cancer (
22,
23 and
24). Hormonal treatments appear to primarily impact fertility due to delayed child-bearing, allowing natural waning of ovarian function (
25).
CONSIDERATIONS FOR WOMEN WHO DESIRE TO HAVE A FUTURE BIOLOGICAL CHILD
Many young women with breast cancer struggle with the competing interests of optimizing personal survival and wishing to have a future biological child (
26). Some choose to modify their expectations regarding future biological children, often considering alternatives such as adoption
(though adoption can be more difficult when a person has a history of cancer). For others, minimizing treatments or pursuing interventions aimed at fertility preservation, or a combination of these, may be appropriate.