(1)
Medical Sciences Division Northern Ontario School of Medicine West Campus, Lakehead University, Thunder Bay, Ontario, Canada
Key Topics
Molecular pathology of renal cell carcinoma (RCC)
Circulating cell-free nucleic acid content as RCC biomarkers
Circulating RCC miRNA biomarkers
Circulating cytokine and angiogenic factors as RCC biomarkers
Circulating RCC cells
Circulating endothelial and hematopoietic lineage cells as RCC biomarkers
Key Points
The incidence of RCC is on the rise due to increasing prevalence of risk factors. Metastatic RCC is almost an incurable disease, and yet 25–30 % of all presenting cases have some aspects of metastasis. Noninvasive screening biomarkers are key to primary and secondary prevention.
While not validated as circulating RCC biomarker, ccfDNA has been extensively investigated in RCC. Of interest, the facile detection should make tracking ctDNA clinically applicable in patient management. Such circulating epigenetic and genetic profiles of RCC are useful in clinical trials as well.
Circulating RCC cell detection has been technically challenging. However, the angiogenic nature of RCC leads to increased circulating cytokine and angiogenic factors as well as endothelial and hematopoietic lineage cells in RCC patients.
10.1 Introduction
Renal cell carcinoma (RCC), the commonest variety of renal cancers, still poses a challenge to oncologists, because metastatic RCC (mRCC) is inevitably a fatal disease. Globally, RCC is about the 12th most diagnosed cancer, matching that of pancreatic cancer. The 2012 global estimated incidence and mortality were 337,800 and 143,406, respectively. In the United States, over 62,700 new cases and 14,240 deaths are expected in 2016. There are geographic variations in the epidemiology of RCC. Age-standardized ratio puts the Czech Republic, Lithuania, Slovakia, and the United States among the countries with the highest incidences, while the Netherlands and Iceland have the lowest rates. While these statistics may not seem alarming, the problem is that RCC incidence has been on the rise since the early 1990s, probably due to enhanced detection by imaging. Similarly, some of the known risk factors are on the rise as well, suggesting the incidence of RCC may mirror these rising risk factors in the future. While the incidence tends to be low in the resource-poor regions of the world, mortality is very high due to late presentation and ineffective management (form lack of resources).
There are myriads of risk factors for RCC. Age is a risk factor for RCC, because it is most commonly diagnosed in people over 64 years of age. It is also racially associated, at least in the United States, being more common in African-Americans and American Indians than the general US population. Dialysis, high blood pressure, and associated administration of diuretics elevate the risk for RCC. Modifiable risks are smoking, obesity, and occupational exposure to substances such as cadmium, herbicides, and organic solvents, especially trichloroethylene. Some genetic diseases elevate the risks for RCC as well. These genetic factors include von Hippel–Lindau (VHL) disease (with VHL mutations), hereditary papillary and renal cell carcinoma (HPRCC – with mutations in MET), hereditary leiomyoma, and renal cell carcinoma (HLRCC – with mutations in fumarate hydratase, SDHB).
The dismal outcome of RCC is due to late diagnosis. There are currently no screening recommendations for RCC, and early lesions cannot be detected by palpation. While imaging can detect small tumors, they are inaccurate at differentiating between benign and malignant lesions. Thus, biomarkers representative of renal tumor biology and hence specific to RCC behavior are needed for accurate early detection, classification, staging, prognosis, and treatment predictions. There are such biomarkers available awaiting validation and translation. However, obtaining tumor tissue for biomarker analyses is invasive and nonrepresentative of tumor heterogeneity. Thus, assaying such biomarkers in body fluids (urine and blood) offers a much better alternative and advancement in renal oncology.
10.2 Molecular Pathology of RCC
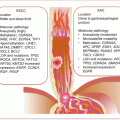
Many (>90 %) cancers of the kidney are of epithelial cell origin (RCC), of which the most frequent (~75 % of cases) are clear cell RCC (ccRCC), followed by papillary RCC (~15 %), and then chromophobe RCC (~5 %). Rare RCC subtypes include collecting duct RCC (~1 %), with the remaining that constitutes <1 % being medullary, mucinous, tubular, spindle cell, Xp11 translocation RCCs, and carcinoma associated with neuroblastoma (Fig. 10.1). These subtypes have different molecular pathology and hence prognosis and targeted therapeutic responses. Oncocytomas are benign renal cell tumors that may not easily be differentiated from RCC on clinical or radiographic evaluations. Hence circulating biomarker studies must await correct histopathologic diagnosis to avoid inclusion of these tumors as malignant renal cancers. The various subtypes also have distinct modes of spread. Clear cell RCC has the fastest growth rate and spreads outside the renal capsule through the vasculature to the lung, liver, bone, and brain. Papillary RCC spreads mostly to lymph nodes, while chromophobe tumors have a higher propensity to spread to the liver than ccRCC.
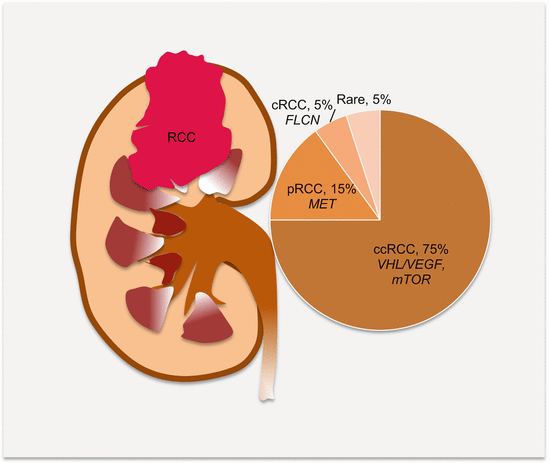

Fig. 10.1
Frequencies of the subtypes of RCC and the commonly associated molecular pathology. ccRCC clear cell RCC, pRCC papillary RCC, cRCC chromophobe RCC
Many RCCs are sporadic; however, ~4 % are associated with hereditary cancer syndromes. Identified are hereditary papillary RCC (type 1 pRCC) and hereditary leiomyosarcoma and RCC (HLRCC; type 2 pRCC). Similar to CRC, these hereditary forms helped inform the molecular underpinnings of sporadic RCC. Indeed, similar molecular pathology is demonstrated for both hereditary and sporadic RCCs, such that it even becomes impossible to separate them based on molecular profiling alone.
10.2.1 Genetics of RCC
The established major signaling pathways deregulated in RCC are the VHL tumor suppressor gene/VEGF, MET membrane-bound tyrosine kinase receptor, mTOR, WNT/β-catenin, and growth factor pathways. But, the best understood molecular pathways in RCC have been elucidated primarily in the major subgroup, ccRCC. Two pathways of clinical relevance (because of the presence of approved targeted agents for members of these pathways) are those tumors that rely on the VHL/VEGF signaling axis and those that employ the mTOR pathway. The vast majority of ccRCC harbors epigenetic and genetic inactivation of the VHL tumor suppressor gene on chromosome 3p25-26. Indeed, VHL inactivation underlies almost all hereditary ccRCC and in ~ 67 % of sporadic cases. Pathophysiologically, loss of VHL functions leads to aberrant induction of HIF gene expression and protein accumulation. An active transcription factor is formed through heterodimerization of HIFα (HIF1α, HIF2α, and HIF3α) subunits with HIF1β (ARNT). In the nucleus, these transcription factors then interact with target gene promoters to induce their expression. Primarily, these are genes induced under hypoxic conditions, which normally promote tumor growth and survival. Additionally induced genes include those for tumor angiogenesis (mostly by HIF2α), glycolytic metabolism (mostly by HIF1α), and cell growth and proliferation.
Different gene alterations cause papillary RCC. The hepatocyte growth factor (HGF) receptor tyrosine kinase, MET, is deregulated through mutations and aneuploidy (e.g., chromosome 7 trisomy) leading to increased expression of activated MET in a vast majority of HPRCC and ~75 % of sporadic pRCC. Similarly, HPRCC is caused by germline mutations in MET. Mutations in fumarate hydratase, the TCA cycle gene, are linked to HLRCC or type 2 pRCC, and finally mutations in folliculin (FLCN) in Birt–Hogg–Dubé syndrome underlie the rare chromophobe RCC.
10.2.2 Molecular Subtypes of RCC
Molecular subtyping has been successful for some cancers such as breast cancer, and this helps guide efficient targeted therapy. Accurate subtyping of the heterogeneous RCCs should improve patient stratification for the several currently available targeted therapies. Some progress has been achieved with protein biomarkers, which associate carbonic anhydrase 9 (CA9) with ccRCC and cytokeratin 7 (CK7) with pRCC. Protein biomarker panels have also proved useful in subgrouping the various RCCs. Multiple gene expression studies further suggest that ccRCC has distinct subtypes (ccA and ccB) with prognostic relevance. But gene expression signatures have failed to reproducibly subclassify all RCCs. Also recognized are The Cancer Genome Atlas (TCGA) subtypes of ccRCC and pRCC associated with overall patient survival.
10.2.3 Personalized Oncology in RCC
The use of each patient’s tumor molecular genetic profile to tailor or customize management is paramount to successful management of RCC, given its molecular pathologic heterogeneity. Pharmaceutical agents targeting the VEGF and mTOR pathways have been developed. Sunitinib (Sutent, Pfizer), sorafenib (Nexavar, Bayer/Onyx), pazopanib (Votrient, GlaxoSmithKline), and axitinib (Inlyta, Pfizer) are TKIs that target VEGFRs, bevacizumab (Avastin, Genentech/Roche) targets the VEGF ligand, while everolimus (Afinitor, Novartis) and temsirolimus (Torisel, Wyeth/Pfizer) interrupt mTOR pathway by blocking the activity of mTORC1.
Circulating RCC signatures have emerged as potential predictors of patients who will benefit from specific targeted therapies (Table 10.1). In metastatic RCC in particular, obtaining tissue biopsy samples from frail patients may not be the optimal sample source, making the minimally invasive sampling of blood desirable. Not only does this represent primary tumor tissue, it equally harbors cells from metastatic deposits, with possible altered genetic signatures. Moreover, blood can easily be sampled serially while the patient is on treatment to monitor progress and treatment decision-making should resistant clones evolve.
Table 10.1
Targeted therapy in RCC
Agent | Target | Clinical indication |
|---|---|---|
Sunitinib | VEGFR | First-line favorable-intermediate risk |
Pazopanib | VEGFR | First-line favorable-intermediate risk |
Bevacizumab | VEGF | First-line favorable-intermediate risk |
Sorafenib | VEGFR | Second line |
Axitinib | VEGFR | Second line |
Temsirolimus | mTORC1 | First-line poor risk |
Everolimus | mTORC1 | Second line |
10.3 Circulating RCC Biomarkers
Circulating RCC biomolecules have been useful as diagnostic, prognostic, and predictive biomarkers. While all biomarkers have been investigated, the clinical potential of cytokine and angiogenic factors is noteworthy. Circulating RCC cells have been difficult to characterize, but circulating endothelial and hematopoietic lineage cells are promising biomarkers of RCC.
10.3.1 Circulating Cell-Free Nucleic Acid Content as RCC Biomarkers
Some attempts are made on evaluating the diagnostic, prognostic, and predictive role of ccfDNA levels in patients with RCC. DNA integrity assay analysis suggests RCC patients harbor larger necrotic genomic fragments than healthy control individuals, whose circulating DNA is derived mostly from smaller fragmented apoptotic DNA. Gang et al. targeted amplification of GAPDH in fragments comprised of 109, 192, 397, and 456 bp in sera to assess DNA integrity in RCC patients [1]. Age and gender were non-confounding variables in both cancer and control groups. For the tumor group, DNA integrity index (DII) had no association with tumor grade. But there was correlation with tumor stage and size. The larger fragments (397 and 456) were undetectable in controls. However, fragment 397 was detectable in 68.2 % of preoperative compared to 31.8 % of postoperative samples, while fragment 456 was detectable in 81.8 % of preoperative compared to 13.6 % of postoperative samples. These findings suggest the tumor cell origin of these ccfDNA fragments. Noteworthy, all samples contained the smaller 109 bp fragment, which is an indication that some ccfDNA in cancer patients are of apoptotic origin (especially in advanced stage cancers). Fragment 397 was detected in 96.2 %, while 456 was observed in 88.5 % of cancer patient samples, suggestive of some diagnostic potential. Hauser et al. used quantitative RT-PCR targeting two fragments of ACTB in 35 RCC patient and 54 healthy control samples [2]. ACTB-106 measured smaller apoptotic fragments, while ACTB-384 detected larger fragments possibly from necrotic cancer cells. The ratio, ACTB-384/ACTB-106, measured the levels of DNA fragmentation. The absolute concentrations of both DNA fragments were significantly higher in cancer patients than controls (p = 0.0003 for ACTB-384, and p = 0.003 for ACTB-106). Expectedly, DII was increased in cancer patients compared to controls (1.07 vs. 0.72, p = 0.04). Both of these studies suggest RCC patients harbor circulating DNA from necrotic cells or other modes of release and less so from apoptotic source.
Other studies have addressed the role of ccfDNA in predicting therapeutic response and postoperative recurrence in ccRCC patients. Feng and colleagues quantified ccfDNA as a therapeutic efficacy response biomarker in patients on sorafenib [3]. Eighteen patients on this therapy had plasma ccfDNA quantified at six different time points (before therapy and at 4, 8, 12, 16, and 24 weeks during treatment). Remission was assessed by CT examination according to RECIST 1.1. Patients in remission or with stable disease had significantly lower plasma ccfDNA from weeks 8 to 24 than patients with progressive disease. Additionally, higher plasma ccfDNA predicted poor outcomes. Using ccfDNA levels at 8 weeks of treatment as a predictor of progressive disease, this assay achieved a sensitivity of 66.7 % and a specificity of 100 %. In order to predict postoperative outcome, ccfDNA was quantified in plasma from 92 ccRCC patients before and after surgery. An increase in ccfDNA was observed in patients with metastatic disease compared to those with localized cancer. Patients with metastatic ccRCC had significantly higher pretreatment ccfDNA (6.04 ± 0.72) than those with localized disease (5.29 ± 0.53, p = 0.017). As will be expected, the levels in cancer patients were much higher than control healthy individuals (0.65 ± 0.29 p < 0.001). Disease recurrence among patients with localized disease is associated with elevated ccfDNA. High pre- and post-surgical ccfDNA levels have in general been associated with disease recurrence.
Circulating mtDNA measurements may help identify people with urologic malignancies. In patients with renal, bladder, and prostate cancers, circulating cell-free mtDNA was amplified targeting two fragments of the MT-RNR1 (12S rRNA: a smaller 79 bp and a larger 220 bp fragments). The contents of both fragments (defined by copy numbers) were significantly higher in cancer patients than in healthy controls, and this achieved a diagnostic sensitivity and specificity of 84 % and 97 %, respectively, for the detection of these malignancies. The copy numbers of both fragments were highest in bladder cancer, followed by RCC and then prostate cancer patients. Circulating mtDNA integrity, defined by the ratio of the larger fragment (220 bp) to the smaller fragment (79 bp), was higher in both RCC and bladder cancer patients than healthy controls and men with prostate cancer, and this elevated levels correlated with pathologic state of RCC and with tumor grade in bladder cancer patients [4]. These findings suggest some tumors release more DNA into the circulation than others.
Free circulating RNA levels are also associated with renal cancers. Feng et al. examined circulating RNA levels using serum samples from patients with renal tumors (RCC and oncocytomas) and healthy controls [5]. The mean concentration was significantly higher in RCC patients (1414.19 ± 91.95 ng/ml) than in those with oncocytomas (560.71 ± 69.54 ng/m; p < 0.0001) and healthy subjects (520.49 ± 39.75 ng/ml; p < 0.0001). The diagnostic AUROCC was 0.956 (95 % CI 0.923–0.989). Serum RNA levels declined in some patients one week after surgery. In another study, this group targeted mRNA of CD133 in peripheral blood as a biomarker of metastasis or predictor of disease recurrence. Blood samples from patients with ccRCC before surgery and healthy controls were analyzed. Patients with metastatic disease had elevated levels of CD133 mRNA (1.546 ± 0.291) than those with localized disease (1.034 ± 0.316, p = 0.022) and controls (0.042 ± 0.028, p = 0.001) Tumor recurrence was associated with increases in CD133 mRNA levels and was predictive at 82.6 % sensitivity and 69.8 % specificity [6]. Could these findings indicate the release of RCC stem cells into circulation? This is important because therapeutic targeting of these cells will immensely augment treatment outcomes in such individuals.
10.3.2 Circulating RCC Epigenetic Biomarkers
A number of studies have explored the clinical utility of DNA methylation in circulation of RCC patents. De Martino et al. examined the prognostic performance of both levels of ccfDNA and specific gene methylation [7]. This study involved consecutively collected preoperative serum samples from RCC patients and patients with benign renal tumors. Cell-free DNA analysis targeted ring finger protein 185 (RNF-185), prostaglandin-endoperoxidase synthase-2 (PTGS2), and CDKN2A. Additionally, CpG island methylations of RASSF1A and VHL were assessed. The diagnostic performances of total ccfDNA targeting the specified genes, and methylation of RASSF1A and VHL, were revealed with AUROCC of 0.755, 0.705, and 0.694, respectively. Noteworthy, VHL methylation was associated more with ccRCC than the other subtypes (p = 0.007). Total ccfDNA levels were higher in metastatic RCC (p < 0.001) and necrotic RCC (0.003) than the other tumors, and these elevated levels conferred poorer disease-specific survival (p < 0.001). In multivariate analysis, however, tumor stage, size, grade, and necrosis (SSIGN) score (p < 0.001), as well as total ccfDNA (p = 0.028), were the significant independent prognostic factors. Another prospective study of serum DNA methylation biomarkers of RCC targeted APC, GSTP1, CDKN2A, RARβ, RASSF1, TIMP3, and PTGS2 DNA in RCC patients and healthy controls [8]. Methylation frequencies varied from 14.3 % for CDKN2A/ARF to 54.3 % for APC gene, and these correlated with increased tumor stage. Methylation of at least one of these genes was observed in as many as 85.7 % of RCC patients. The methylation of all the genes except CDKN2A and TIMP3 was significantly observed in all patients. While these genes showed very high specificities for RCC detection, the sensitivities were very low. However, as a panel, they performed much better. For example, methylation of APC, PTGS2, and GSTP1 achieved a sensitivity of 62.9 % and a specificity of 82 %.
10.3.3 Circulating RCC Genetic Biomarkers
Studies on circulating genetic alterations in RCC have focused mainly on microsatellite alterations (MSAs). Nine polymorphic markers on eight chromosomal regions were used to interrogate MSAs in serum samples from patients with RCC. Allelic imbalance (AI) was detected at a rate of 74 % of these patients. However, by increasing the number of markers to 20, the detection of AI was increased to 87 %. Most alterations occurred on chromosomes 3p and 5q. The AI was mostly associated with advanced stage carcinomas [9]. In another study, plasma DNA concentration and MSA were examined in patients with ccRCC. Plasma DNA concentration was significantly higher in patients than controls, and the levels decreased after surgery. MSA was found in 75.8 % of tumor tissues and 55.6 % of plasma samples. Increasing plasma DNA concentration and MSAs were predictive biomarkers of recurrence [10].
Because as many as 80 % of ccRCCs have chromosome 3p losses, four polymorphic markers (D3S1307, D3S1560, D3S1289, and D3S1300) on chromosome 3p were used to study tissue and plasma samples from ccRCC patients. Sixty three percent of tissue samples had at least one MSA, of which 35 % were in corresponding plasma, and the tissue and plasma MSAs were identical [11]. Gonzalgo et al. evaluated the prognostic value of MSA using 28 microsatellite markers in a prospective study [12]. Serum, urine, and tissue samples were all examined. Patients had surgery and were followed for 2 years. The frequency of preoperative serum MSA was a significant prognostic indicator of disease recurrence. Preoperative serum MSA is useful in the detection of RCC, and the frequency can identify high-risk groups with possible recurrence after surgery [12]. Thus, MSAs are common in RCC and can be detected in circulation. The potential clinical utility requires further investigation.
10.3.4 Circulating RCC Noncoding RNA Biomarkers
RCC is associated with deregulated expression of miRNAs that may have biologic implications in RCC progression and metastasis. Oncogenic RCC miRNAs include miR-21, miR-23-3p, miR-34, miR-100, miR-142-3p, miR-155, miR-185, miR-210, and miR-224. MiR-21 and miR-23-3p target PTEN in RCC and are upregulated. RCC tumor suppressormirs include miR-99a, miR-138, miR-141, miR-143, miR-145, miR-149, miR-192, miR-194, miR-200c, miR-205, miR-215, and miR-1285. Targets of miR-192, miR-195, and miR-215 are ZEB2 and MDM2 involved in EMT.
Studies of circulating miRNAs in RCC have been addressed with some potential leads in regard to novel biomarkers. The 2011 study by Wulfken and colleagues uncovered 109 miRNAs elevated in sera from patients with RCC, of which the levels of 36 were concordant with alterations in tissue samples. Validation analysis identified only miR-1233 as having some diagnostic utility with a sensitivity of 77 % but a dismal specificity of 37.6 % [13]. In another study, Redova et al. found 30 miRNAs to be deregulated in sera from patients with RCC, of which 19 were upregulated and 11 were downregulated [14]. In a validation study, only miR-378 (upregulated) and miR-451 (downregulated) were able to separate RCC patients from controls. Used in combination as diagnostic biomarkers, this panel achieved a sensitivity of 81 % and a specificity of 83 %. The elevated HIF associated with VHL mutations in RCC controls miR-210 expression. Consistently, the levels of miR-210 are increased in RCC tissue samples, and this is explored in circulation as well. Sera from RCC patients have elevated levels of miR-210, and these high levels significantly fell a week following nephrectomy (p = 0.001). As a diagnostic biomarker, the elevated miR-210 levels had a sensitivity and specificity of 81 % and 79.4 %, respectively [15]. Another study confirmed the elevated levels of serum miR-210 in ccRCC samples compared with paired normal renal tissue. In this cohort, serum levels were equally higher in ccRCC patients than healthy controls (p = 0.001). As a diagnostic biomarker, this single serum assay achieved a sensitivity of 65 % and a specificity of 83 % with AUROCC of 0.77 (95 % CI 0.65–0.89) [16]. Serum levels of miR-193a-3p, miR-362, and miR-572 were significantly elevated, while miR-28-5p and miR-378 were decreased in RCC patients compared to controls [17]. The diagnostic AUROCC for this panel of five miRNAs was 0.807 and 0.796, respectively, in training and validation sample sets. Of interest, early stage I patients were detected at a sensitivity of 80 %, specificity of 71 %, and AUROCC of 0.807.
The prognostic role of circulating miR-221 and miR-222 in RCC has been explored. These miRNAs are elevated in RCC tissue samples and are implicated in disease metastasis. The circulating levels are equally high in RCC patients than healthy control individuals (p = 0.044), and miR-221 levels were associated with disease metastasis (p = 0.001). Higher levels of miR-221 conferred decreased OS (48 vs. 116 months, p = 0.024). Cox regression analysis indicated that higher circulating miR-221 was associated with cancer-specific death (HR, 10.7, 95 % CI, 0.13–85.65), p = 0.026) [18]. Of interest, other miRNAs have been demonstrated to be elevated in circulation of patients with RCC, but were of no clinical value in this series. These include miR-21, miR-26a-2-3p, miR-191, miR-337-3p, and miR-378. Note, however, that miR-378 is demonstrated to have some diagnostic value. Sensitive technologies and standardized assays will reveal the true value of these elevated miRNAs in circulation of RCC patients. Finally, extracellular vesicles of RCC stem-/tumor-initiating cells are enriched with miR-200c, miR-92, and miR-141 that may shuttle in circulation to participate in preconditioning of metastatic niches [19].
10.3.5 Circulating RCC Protein Biomarkers
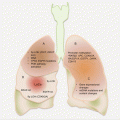
Serum proteins have been useful in clinical management of RCC patients. Specifically, the altered levels of cytokine and angiogenic factors (CAFs) have proven useful as prognostic and predictive biomarkers (Fig. 10.2). Adding to this biomarker pool are novel proteins and peak signatures discovered from MS analysis of serum samples.
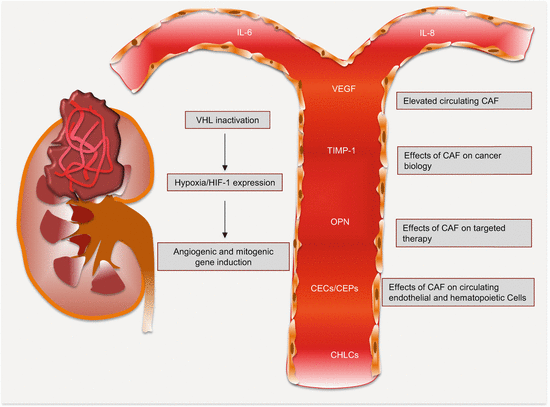

Fig. 10.2
Circulating cytokine and angiogenic factors and their effects in RCC. The differential levels between cancer patients and healthy controls hold biomarker potential
10.3.5.1 Serum Proteins as RCC Biomarkers
RCC Diagnostic Serum Protein Biomarkers
The need for early detection biomarkers for RCC is certainly urgent since the cure rate can be as high as >90 % for pathologic stage T1. To this end, Su Kim and colleagues embarked on analysis of three potential biomarkers (NNMT, LCP1, and NM23A) for early detection of RCC [20]. In this assay, plasma levels of all three biomarkers were significantly elevated in RCC patients compared to healthy controls and people with benign tumors (p < 0.0001). Remarkably, in a blinded validation study encompassing 175 controls and 114 patients with diverse subtypes of renal cancer, NNMT as a single biomarker and the three as a panel achieved a diagnostic accuracy of 0.913 and 0.932, respectively. At a set of 90 % specificity, NNMT and the panel had a sensitivity of 71.9 % and 95.7 %, respectively. The PPV (87.2 %) and NPV (97 %) were equally impressive. Noteworthy, these biomarker performances were independent of RCC subtypes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree