INCIDENCE AND DIAGNOSIS
The American Cancer Society predicts that in 2015 there will be over 64,000 new cases of renal neoplasms in the United States and that 14,000 patients will die as a consequence of disease progression (1). Renal cell carcinoma (RCC) is the most common histology found in kidney tumors, with clear cell RCC (ccRCC) being the most common histologic subtype (Fig. 35-1). Non–clear cell RCC (nccRCC) subtypes include chromophobe, papillary, oncocytoma, collecting duct carcinoma (CDC), renal medullary, translocation, and unclassified RCC.
FIGURE 35-1
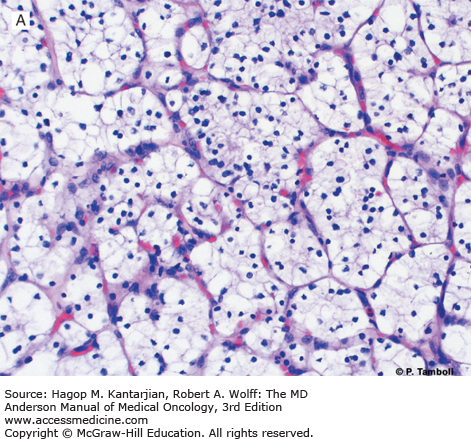
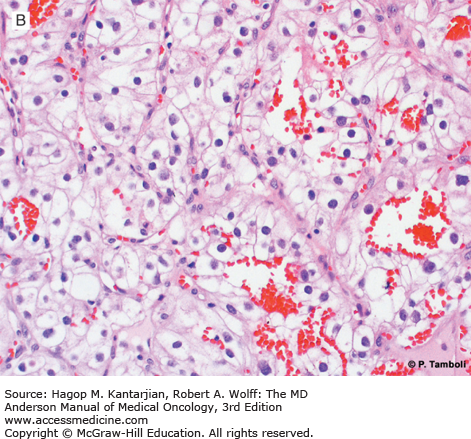
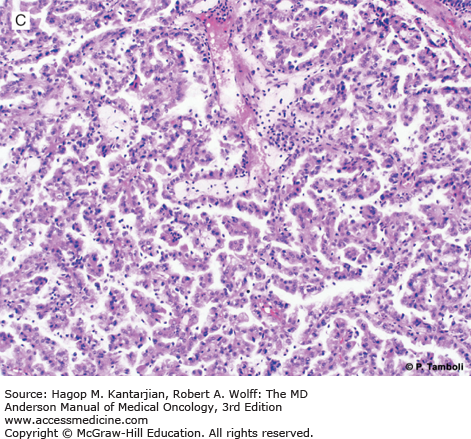
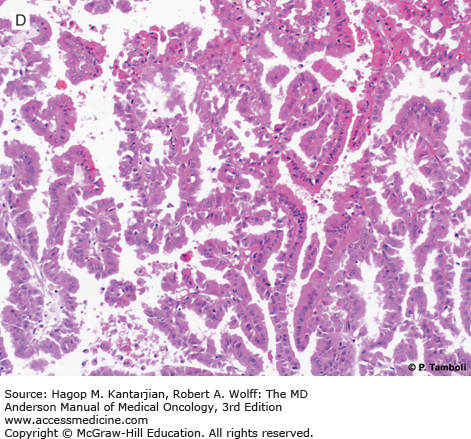
Photomicrographs of clear cell (conventional) renal cell carcinoma (RCC) with low-grade (A) and high-grade (B) nuclear features. Photomicrographs of a type 1 papillary RCC (C) showing papillae lined by short cuboidal cells and a type 2 papillary RCC (D) showing papillae lined by tall columnar cells, with eosinophilic cytoplasm and high-grade nuclear features. (Used with permission from Pheroze Tamboli, MD.)
Work by Chow et al (1) found the worldwide incidence of RCC appearing to plateau after a steady increase over several decades. To examine the incidence in the United States, the group used the database of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program to track patients with a diagnosis of kidney cancer from 1977 to 2006. The rate of localized cancer detection has continued to increase, whereas the rates of regional, metastatic, and unstaged RCC have declined. In this work, the mortality rate associated with RCC appears to begin declining in the early 2000s across both gender and racial lines. Although the direct causal relationship for the decline in RCC mortality in this study is unclear, early detection in the era of computed tomography (CT) imaging may be contributing to this finding.
STAGING, EPIDEMIOLOGY, AND RISK FACTORS
The American Joint Commission on Cancer staging schema for RCC was updated in 2010. Major staging categories are as follows: stage 1: T1 tumors that are 7 cm in maximum diameter or less and are confined to the kidney; stage 2: T2 tumors that exceed 7 cm in diameter but are confined to the kidney; stage 3: T3 tumors that demonstrate extracapsular invasion into the perinephric adipose tissue or renal sinus or extend into the renal vein or inferior vena cava (stage 3 also includes tumors with regional lymph node metastasis); and stage 4: extension of the primary tumor into the ipsilateral adrenal gland or beyond Gerota’s fascia or distant metastases (2).
The link between germline genetic mutations and the development of RCC is well established and applies to a small but biologically important subset of RCC cases (3). Although these genetic alterations certainly play an important role in the biology of RCC in both familial and sporadic cases, there are also some environmental factors that contribute to the risk of developing a renal neoplasm. Smoking has long been linked to an increase in the risk of developing RCC, in addition to its association with multiple other malignancies (4). As is the case with other malignancies, cessation of smoking can be associated with a diminution in the risk of developing RCC (5). Interestingly, this diminution in risk appears to be slower and requires a significantly longer time to reach baseline than that seen with other malignancies, such as lung cancer.
Obesity or increased body mass index (BMI) has also been linked to increased risk of developing RCC. Several studies have demonstrated a higher incidence of obesity or increased BMI in patients with RCC, suggesting an epidemiologic linkage (6). As is the case in most retrospective epidemiologic studies, these observations are confounded by other associated variables, including diet, occupational history, and smoking. In a report by Kamat et al., overweight and obese patients had a more favorable prognosis following surgery than did patients with a lower BMI (7). Additional possible risk factors identified retrospectively in epidemiologic studies include diabetes, hypertension, and treatment with diuretics; in addition, certain diets have been linked to higher or lower risk than the general population.
PROGNOSTIC FACTORS, PATHOLOGY, AND MOLECULAR MARKERS
The natural history is variable, depending on tumor genetic factors, the general medical condition of the patient, and factors such as angiogenesis and immune response. Patients who undergo nephrectomy for localized disease remain at risk of recurrence for many years and require appropriate counseling and surveillance.
The traditional measures of performance status, tumor stage, and tumor grade each demonstrate good correlation with clinical outcome (8). Integration of these parameters and other clinical prognostic variables allows the classification of patients into groups with statistically significant differences in survival. There are a variety of clinical, pathologic, and molecular features that have been proposed and studied as prognostic factors (Table 35-1) (9,10). As the genomic landscape continues to be more clearly defined in ccRCC and the genetic drivers of metastasis are identified, more refined putative biomarkers are needed to more effectively treat this heterogeneous disease (11,12).
| Patient- or Treatment-Related Factors | Laboratory Studies | Tumor-Related Factors | Molecular Markers |
|---|---|---|---|
| Performance status | Lactate dehydrogenase | Site and/or number of metastatic sites | VHL mutation or hypermethylation |
| Age, gender, race | Alkaline phosphatase | Disease-free interval | Carbonic anhydrase IX expression |
| Symptoms: weight loss, fatigue, pain, loss of appetite, fever | Calcium | Metastasis-free interval | Phospho-extracellular signal regulated kinase (pERK) |
| Overweight | Albumin | Tumor burden | Cytoplasmic mTOR staining |
| Prior nephrectomy | Liver dysfunction | Histologic type | PTEN deletion |
| Prior therapy | Anemia Neutrophilia | Sarcomatoid dedifferentiation | p53 overexpression |
| Thrombocytosis | Ploidy | MMP-2 and MMP-9 overexpression BAP1 and PBRM1 mutation |
In 1999, Motzer et al identified five risk factors (elevated serum corrected calcium level, anemia, lactate dehydrogenase >1.5× the upper limit of normal [ULN], Karnofsky performance score [KPS] <80, and primary tumor in place) that segregated patients into three risk groups (good, zero factors; intermediate, one to two factors; and poor, three or more factors) (13). In 2002, an updated version, now referred to as the Memorial Sloan-Kettering Cancer Center (MSKCC) risk criteria (also referred to as the “Motzer criteria”), was published with the original four risk factors, with the fifth being treatment of interferon within 1 year of diagnosis (14). For clinical trial purposes, the fifth factor is often considered time to initiation of systemic therapy within 1 year from initial diagnosis of RCC. With the introduction of targeted therapy, the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) was formed, and a modified prognostic scoring system was put forth in 2009 and externally validated in 2013 (15,16). The IMDC prognostic scoring system (also referred to as the “Heng score”) has six risk factors (elevated serum corrected calcium level, anemia, KPS <80, systemic treatment for metastatic disease within 1 year, absolute neutrophil count >ULN, and platelet count >ULN). Similar to the MSKCC scoring system, good risk is defined as zero risk factors, intermediate risk as one to two risk factors, and poor risk as three or more risk factors. Although other systems have been proposed, the MSKCC and IMDC remain the most widely used (Table 35-2).
| MSKCC 2002 Factors (1 point each) | Risk Group (points) | Overall Survival (months) | IMDC or Heng Score (1 point each) | Risk Group (points) | Overall Survival (months) |
|---|---|---|---|---|---|
| Anemia | Good (0) | 30 | Anemia | Good (0) | 43 |
| Elevated calcium | Elevated calcium | ||||
| KPS <80 | Intermediate (1-2) | 14 | KPS <80 | Intermediate (1-2) | 22.5 |
| Metastatic Tx within 1 year of Dx | Metastatic Tx within 1 year | ||||
| LDH >1.5× ULN | Poor (3-5) | 5 | Platelet count >ULN | Poor (3-6) | 7.8 |
| Neutrophil count >ULN |
Non–clear cell histology has been associated with lower response to systemic therapy (17). In an attempt to improve on the MSKCC clinical prognostic model, we investigated the role of cytokines and angiogenic factors (CAFs) in serum of patients treated with interferon-α (IFN-α) and found elevated baseline levels of interleukin (IL)-5, IL-6, IL-12p40, and vascular endothelial growth factor A (VEGFA) to be independent risk factors associated with inferior survival. Incorporating the CAF model with the MSKCC clinical model improved the concordance index for predicting overall survival (OS). Patients with three or more of the four CAFs or with MSKCC poor-risk status had a median OS of 9 months, compared with a median OS of 32 months for patients with two or fewer CAFs (18). Similar efforts have been undertaken to develop serum and plasma-based prognostic and predictive biomarkers in individuals who received antiangiogenic therapy (19,20).
There are several major histologic subtypes in RCC, including clear cell, papillary, and chromophobe RCC (see Fig. 35-1). Historically, patients with nccRCC variants except chromophobe do poorly once they develop metastatic disease, mainly due to the dearth of effective systemic therapy.
The VHL gene product regulates the hypoxia-induced pathway and is commonly mutated in ccRCC (11). The hypoxia-induced pathway leads to the activation of survival genes that mediate glucose transport, proliferation, angiogenesis, and pH regulation and is also implicated in the other tumor types. In a study of 187 patients undergoing nephrectomy for ccRCC in Japan, mutation or hypermethylation of the VHL gene was found in 58% of tumors, and was associated with significantly improved disease-free and cancer-specific survival in patients with organ-confined tumors (n = 134), but not in those with stage IV disease at the time of nephrectomy (n = 53) (21). VHL loss occurs early in the development of clear cell carcinoma (12), potentially identifying a subset of patients who do well after surgery.
In ccRCC, loss of the short arm of chromosome 3 was the most common genetic alteration identified in The Cancer Genome Atlas (TCGA) analysis. The most common genetic mutations related to this loss include VHL, SETD2 (a histone methyltransferase), BAP1 (histone deubiquitinase), and PBRM1 (part of chromatin remodeling complex), and all lie on chromosome 3p (11). The University of Texas Southwestern group identified BAP1 mutations to be associated with poor survival, with a median OS of 4.6 years from nephrectomy in patients who present with nonmetastatic disease compared with a median OS of 10.6 years in patients whose tumors harbor PBRM1 mutations. The investigators validated these findings from tumor specimens included in the TCGA analysis (22).
MANAGEMENT OF NONMETASTATIC RENAL CELL CARCINOMA
With the introduction of improved cross-sectional imaging including CT and magnetic resonance imaging (MRI), the detection of renal masses has increased substantially. The ability to characterize renal masses as benign or malignant appearing has also evolved with improved technology and the use of specific contrast-enhancing sequences. The management of small renal masses (<4 cm) will not be extensively covered in this chapter but includes options such as active surveillance, partial nephrectomy (PN), radical nephrectomy (RN), or thermal ablation depending on factors including patient comorbidities and surgical expertise (23). Current surgical approaches to both PN and RN include open, laparoscopic, and robotic-assisted techniques (24,25). Loss of nephrons associated with RN compared to PN increases patient risk for development of chronic kidney disease (CKD)(26). In the general population, CKD is associated with increased risk of mortality and cardiovascular disease. Retrospectively, patients treated with RN were found to have higher all-cause mortality and an increased incidence of cardiovascular events (27).
With the introduction of robotic partial nephrectomy, the warm ischemia and suturing times are reduced compared to laparoscopic PN even among experienced laparoscopic surgeons (28). The management algorithm of potential tumors amenable to PN continues to evolve and should take into account the health of the contralateral kidney, familial cancer syndromes leading to multiple renal masses, proximity to the hilum and vascular structures, baseline kidney function, and surgeon experience and volume.
With larger, more locally advanced tumors requiring nephrectomy, the decision to perform a lymph node dissection remains an area of debate. In the European Organization for Research and Treatment of Cancer (EORTC) 30881 trial, 772 patients were randomized between lymph node resection or no resection during nephrectomy procedure. The majority of patients had ≤T2 disease (29). The study found no difference in recurrence-free survival or OS. However, other series have found the presence of lymph node positivity to be directly correlated with increasing T stage. As a result, at the University of Texas MD Anderson Cancer Center (MDACC) when patients have radiographic evidence of lymph node involvement or T stage ≥T3a, a therapeutic or staging lymph node dissection is typically undertaken.
The decision to perform ipsilateral adrenalectomy as part of the standard RN procedure has evolved. In a retrospective review of the literature, O’Malley et al estimated a negative predictive value of 96% when cross-sectional imaging of the ipsilateral adrenal gland was compared to surgical pathology (30). Unless there is evidence of involvement either by direct extension, radiographic evidence of metastatic spread of disease, or concern for involvement during gross inspection during surgery, the ipsilateral adrenal gland is typically spared.
Locally advanced tumors staged as T3b or higher require considerable institutional and surgeon experience in the management of these patients and will not be covered here in detail.
METASTATIC DISEASE WITH CLEAR CELL HISTOLOGY
Renal cell carcinoma remains a relatively rare malignancy where addressing the primary tumor in selected patients with metastatic disease leads to an improvement in OS (31,32). In a pooled analysis of the results of the Southwest Oncology Group (SWOG) 8949 and EORTC 30947 studies, improvement in OS favored the nephrectomy arm, with a median survival of 13.6 months versus 7.8 months for those randomized to the surgical arm (33). Because the initial studies compared patients treated with IFN-α with or without cytoreductive nephrectomy, the question of benefit of cytoreductive nephrectomy in the era of targeted therapy has reemerged. In a retrospective analysis, the IMDC found that patients treated in the targeted era appear to benefit from cytoreductive nephrectomy if they have three or fewer prognostic risk factors as outlined in Table 35-2, whereas those with four or more factors do not appear to benefit (34). Two ongoing prospective trials, SURTIME (NCT01099423) and CARMENA (NCT0093033), are attempting to address two questions regarding targeted therapy and cytoreductive nephrectomy. The SURTIME trial is investigating whether cytoreductive surgery is better performed before or after first-line sunitinib. The CARMENA trial is an EORTC effort investigating whether cytoreductive nephrectomy is beneficial in patients treated with sunitinib. Based on the available data and our institutional experience, patients at MDACC with synchronous metastatic disease who have a good performance status, a good or intermediate prognosis based on MSKCC or IMDC score, ccRCC, and a sizable renal primary compared to metastatic disease burden are generally offered upfront cytoreductive nephrectomy.
Patients who present with metastatic disease and primary tumor in situ offer an ideal presurgical setting for clinical and translational research studies (35). Retrospectively, patients at MDACC treated with targeted agents prior to cytoreductive nephrectomy compared to upfront cytoreductive nephrectomy experienced similar rates of serious adverse events (36). With the ability to analyze treated tissue paired with time blood samples and clinical and radiographic parameters at defined intervals, presurgical clinical trials offer an opportunity to enhance our understanding of metastatic RCC and determinants of response and resistance to targeted and immunotherapeutic agents.
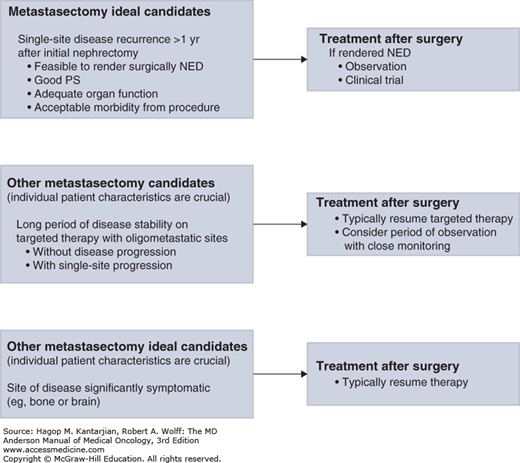
In carefully selected patients, metastasectomy plays an important role in the multidisciplinary treatment of patients with metastatic RCC (mRCC). Retrospective series have found survival benefit in resecting oligometastatic disease from multiple sites. Interpretation of these results is always limited due to the highly selected and retrospective nature of these studies. However, appropriate patient selection, complete resection of disease, and metachronous single site or oligometastatic disease positively impact patient outcomes. In a pooled retrospective analysis from three centers including patients from MDACC, 22 patients who had received targeted therapy underwent consolidative metastasectomy with an acceptable postoperative complication rate. After 108 weeks of follow-up, 11 patients remained disease free, and the median time to resumption of targeted therapy was 55 weeks (37). An ongoing randomized phase II study (NCT01575548) is addressing the role of pazopanib versus placebo in patients with no evidence of disease after metastasectomy. Further treatment decisions are based on the timing of recurrence, sites of recurrence, and individual patient characteristics. At MDACC, we strongly believe a multidisciplinary approach at high-volume centers is required to optimally manage these patients.
An algorithm to select candidates for metastasectomy and for the approach to postmetastasectomy systemic treatment is shown in Fig. 35-2.
SYSTEMIC THERAPY
Until the introduction of targeted therapy (discussed in more detail in the later section on targeted therapy), metastatic resection and cytokine therapy remained the only viable treatment options in mRCC. Interferon-α and IL-2 have been extensively evaluated over the past three decades. In a randomized phase II study from MDACC, treatment with low-dose IFN-α-2b compared to intermediate-dose IFN-α-2b resulted in no significant differences in progression-free survival (PFS) or OS, but patients had an improved quality of life while on low-dose therapy (38). Sustained complete remissions with IFN-α are rare (1%-2%), and with the relatively low response rate of approximately 7%, IFN-α monotherapy never received US Food and Drug Administration (FDA) approval for treatment of mRCC and is no longer considered as a single agent for mRCC at MDACC.
High-dose IL-2 (HD IL-2) received FDA approval in the treatment of mRCC in 1992 based on the results of several phase II trials that found an overall response rate of 15% to 20% and a durable response in the majority of patients who achieved a complete response. A phase III trial comparing HD IL-2 with an outpatient low-dose IL-2 plus IFN-α regimen yielded a higher response rate to HD IL-2 of 23% versus 10% but no statistically significant differences in PFS and OS. However, durable responses of greater than 3 years were seen in 7% of those treated with HD IL-2 versus 0% of patients in the other arm, supporting HD IL-2 as a viable standard-of-care option for patients who are candidates for this therapy (39). Although the management of toxicity related to the delivery of HD IL-2 is challenging, high-volume centers have published a toxic death rate of less than 1%, with response rates of roughly 15% to 20% and durable remissions in the range of 5% to 7% (40,41). Given the ability to produce a sustained remission with likely cure in a small subset of patients, at MDACC, we offer frontline HD IL-2 therapy to patients with excellent performance status, previous nephrectomy, limited or no comorbidities, and low-volume metastatic disease burden, especially lung-only disease.
Over the last decade, seven agents have been FDA approved for the treatment of mRCC. Of these agents, five are vascular endothelial growth factor (VEGF) pathway–blocking agents, with four being within the small-molecule tyrosine kinase inhibitor family and one, bevacizumab, being a monoclonal antibody directed against VEGF on the cell surface. The remaining two agents are mammalian target of rapamycin (mTOR) inhibitors. These agents target both the tumor cells and the tumor microenvironment.
The presence of a VHL mutation in up to 80% of patients with ccRCC and the resultant increased production of angiogenic factors has made this axis the most exploited treatment target. A number of agents that target either VEGF or its receptors (VEGFR) were FDA approved in the past 10 years (Table 35-3).
| Agent | Year FDA Approved | Trial Design | Setting | No. of Patients | MSKCC Risk (%) | Median PFS (months) | Median OS (months) |
|---|---|---|---|---|---|---|---|
| Sorafenib | 2005 | Sorafenib vs placebo | Cytokine failures ccRCC | 903 | Good: 50 Int: 49 Missing: 1 | 5.5 vs 2.8 | 19.3 vs 15.9 (NS) |
| Sunitinib | 2006 | Sunitinib vs IFN | Frontline ccRCC | 750 | Good: 34 Int: 59 Poor: 7 | 11.0 vs 5.0 | 26.4 vs 21.8 (P = .051) |
| Temsirolimus | 2007 | Temsirolimus vs temsirolimus plus IFN vs IFN | Frontline Any histology | 626 | Good: 0 Int: 26 Poor: 74 | 5.5 vs 4.7 vs 3.1 | 10.9 vs 8.4 vs 7.3 |
| Everolimus | 2009 | Everolimus vs placebo | Sorafenib or sunitinib failures ccRCC | 416 | Good: 28.5 Int: 56.5 Poor: 15 | 4.9 vs 1.9 | 14.8 vs 14.4 |
| Bevacizumab plus IFN-α | 2009 | Bevacizumab plus IFN vs IFN plus placebo | Frontline ccRCC | 649 | Good: 28 Int: 56 Poor: 9 Unknown: 8 | 10.2 vs 5.4 | 23.3 vs 21.8 |
| Bevacizumab plus IFN vs IFN | Frontline ccRCC | 732 | Good: 26 Int: 64 Poor: 10 | 8.5 vs 5.2 | 18.3 vs 17.4 | ||
| Pazopanib | 2009 | Pazopanib vs placebo | Frontline and cytokine failures ccRCC | 435 | Good: 39 Int: 54 Poor: 3 | 11.1 vs 2.8 | 22.9 vs 20.5 |
| Axitinib | 2012 | Axitinib vs sorafenib | Second line ccRCC | 723 | Good: 28 Int: 37 Poor: 33 | 6.7 vs 4.7 | 20.1 vs 19.2 |
Bevacizumab is a humanized recombinant anti-VEGF antibody. Two large randomized phase III studies demonstrated an improved PFS in patients who received a combination of bevacizumab plus IFN-α, when compared to IFN-α alone in patients with mRCC who had not received prior systemic therapy (42,43). The AVOREN study compared patients treated with the combination of bevacizumab plus IFN versus placebo plus IFN and found that the bevacizumab combination resulted in a superior median PFS of 10.2 months compared to 5.4 months. The Cancer and Leukemia Group B (CALGB) 90206 study yielded a median PFS of 8.5 months for the combination arm versus 5.2 months for IFN monotherapy, a difference that was statistically significant. Of note, the OS for both studies was numerically superior in the bevacizumab-containing arm but did not reach statistical significance. Bevacizumab plus IFN was FDA approved for advanced RCC in 2009.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree