Keywords
chemotherapy, granulosa cells, oocytes, primary ovarian insufficiency, radiotherapy
2.1
Introduction
More than 75% of children treated for malignant disease will be alive 10 years following initial diagnosis in the UK. In addition, many women of reproductive age will undergo curative treatment for malignant disease. An understanding of the impact that these treatments will have on their future reproductive health is critical in order to inform both treatment choices and counselling with respect to educational, employment and family-planning decisions.
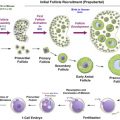
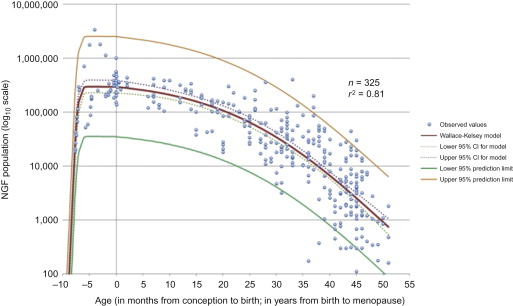
The number of primordial follicles in the human ovary is highest at 5 months gestation, following which the rate of loss progressively accelerates, particularly in the 10 years prior to the menopause ( Figure 2.1 ). Radiotherapy and chemotherapy both have an impact on follicle numbers and, as such, can affect future female fertility. This includes acute ovarian failure, or premature ovarian insufficiency (POI) (either loss of menses as in acute ovarian failure or menstruation within the context of a damaged ovary). Some studies have focused on the incidence of acute ovarian failure as a marker of impact on fertility, but other mechanisms, particularly later POI, significantly affect female fertility following cancer treatment. In healthy women, fertility is lost one decade before natural menopause, with a decline over the preceding decade. Therefore, in cancer patients who experience POI, it is likely that their fertility decreases markedly several years before this. As such, the best marker of the reproductive capacity following cancer treatment is fertility itself rather than incidence of POI. Because a range of terms are used in the literature for describing ovarian damage, the terminology used in the original report will be used here.
The cancers whose treatment most often results in female infertility are leukaemia, Hodgkin lymphoma, non-Hodgkin lymphoma, breast carcinoma, sarcomas and germ cell tumours. The incidence of female infertility within each cancer type depends upon the age at diagnosis, whether surgery or radiotherapy affects the reproductive organs (including the hypothalamic–pituitary axis) and the nature of the chemotherapy that is used. The combination of chemotherapy and radiotherapy is more likely to have a significant impact on fertility but, for a given individual, precisely predicting this impact is difficult, particularly given the large inter-patient variation in ovarian reserve (discussed further in Chapter 3 ). Over the course of the last decade there has been increasing use of targeted biological therapies to treat cancer. There has been less experience with these and the long-term consequences of their exposure on female fertility are unclear. There is some suggestion that bevacizumab (one of the more common biological agents, used in the treatment of colorectal, lung, breast, ovarian, cervical, brain and renal cancer) can cause permanent ovarian failure.
A retrospective questionnaire-based survey of 5-year survivors from the Childhood Cancer Survivor Study demonstrated that they had a decreased incidence of becoming pregnant compared to female siblings (OR, 0.81; 95% CI, 0.73–0.90, p<0.001). In this study, acute ovarian failure occurred in 6.3% of eligible survivors. High-dose radiation (>10 Gy) and exposure to alkylating agents were all significant risk factors for acute ovarian failure. In addition, premature non-surgical menopause occurred in 8% of patients compared to 0.8% of siblings. Risk factors for premature menopause include: attained age, exposure to increased dose of radiation, exposure to alkylating agents and high-dose chemotherapy. The British Childhood Cancer Survivor study retrospectively assessed the fertility of 5133 females by questionnaire. The number of live births was two-thirds of that expected (O/E, 0.64; 95% CI, 0.62–0.66) and was lowest amongst survivors treated with brain (O/E, 0.52; 95% CI, 0.48–0.56) and abdominal irradiation (O/E, 0.55; 95% CI, 0.50–0.61).
Here we describe the impact of radiotherapy and chemotherapy on future female fertility and then summarize the mechanisms of action of different classes of chemotherapy drugs in order to form the context for the discussion of the specific effects of these agents on the ovary in subsequent chapters.
2.2
Impact of Radiotherapy on Future Female Fertility
In female premenopausal cancer patients, any radiotherapy plan that affects the ovaries (whether that is as part of pelvic, abdominal or whole-body irradiation) will have an impact on fertility. The extent of this impact is dependent on patient age, dose, radiotherapy field and fractionation schedule. Radiotherapy-induced primordial follicle oocyte loss depends upon the size of the oocyte pool. As such, the younger the patient is at the time of radiotherapy the later the onset of POI (for a given dose of irradiation). Irradiation of the uterus can result in decreased fertility probably because of effects on the uterine musculature and vasculature, although the exact mechanisms and the extent of this impact (as discrete from the effects of ovarian irradiation) are poorly defined. Cranial irradiation can also have a profound impact on fertility through its effect on circulating gonadotropin levels. However, use of exogenous gondotropins or gonadotropin-releasing hormone can be used to treat this cause of radiation-induced infertility.
Multivariate analysis of the female fertility rate in the Childhood Cancer Survivor Study showed that it was significantly decreased in women previously exposed to a hypothalamic/pituitary radiotherapy dose ≥30 Gy (RR, 0.61; 95% CI, 0.44–0.83) or an ovarian/uterine dose of >5 Gy (RR, 0.56 for 5–10 Gy; 95% CI, 0.37–0.85; and RR, 0.18 for >10 Gy; 95% CI, 0.13–0.26). In a more specific analysis of the same patient group, multivariable analysis revealed that female cancer survivors had a decreased chance of pregnancy with hypothalamic/pituitary doses >22 Gy compared to those with no hypothalamic/pituitary radiotherapy. There is no indication regarding the extent to which this is related to the direct impact of hypothalamic/pituitary radiation and the extent to which this is related to other factors, e.g., impact on growth, progression through puberty, etc. The potential for selection bias is a factor that must be borne in mind when interpreting the results of these questionnaire-based studies.
2.3
Impact of Chemotherapy on Future Female Fertility
In a retrospective survey-based analysis of reproductive-age Californian women treated with chemotherapy for cancer, Letourneau et al. found the incidence of acute ovarian failure to be 10% in non-Hodgkin lymphoma, 9% in breast cancer, 8% in Hodgkin lymphoma, 5% in gastrointestinal cancers and 3% in leukaemia. The proportion of women experiencing acute ovarian failure significantly increased with older age at diagnosis. For patients whose menses returned within 12 months of treatment, there was an association between early menopause and younger age at diagnosis.
Multivariate analysis of the female fertility rate in the Childhood Cancer Survivor Study showed that patients who received higher doses of alkylating agents were less likely to become pregnant compared to selected female siblings.
A largely retrospective study in Asian non-metastatic breast cancer patients showed that patient age at chemotherapy treatment was the only statistically significant factor in determining the incidence of chemotherapy-induced ovarian failure and reversible amenorrhoea. In this study, the overall incidence of amenorrhoea was high, both at the end of chemotherapy (93%) and 12 months following chemotherapy.
A retrospective analysis of a large cohort of Hodgkin lymphoma survivors treated in the European Organisation for Research and Treatment of Cancer (EORTC) and Group d’Etude des Lymphomes de l’Adulte trials between 1964 and 2004 identified premature ovarian failure in 60% of patients (95% CI, 41–79%). The incidence was, however, only 3% (95% CI, 1–7%) after non-alkylating agent chemotherapy regimes. There was a linear relationship between premature ovarian failure and both use of alkylating agent chemotherapy and age at treatment. In patients receiving non-alkylating chemotherapy, while the incidence of premature ovarian failure was only 3% if treated before the age of 32 years, it was 9% if treated after the age of 32. If menstruation returned after treatment then the premature ovarian failure risk was independent of age at treatment in this study (in contrast to the study by Letourneau et al. described above). In terms of the impact on subsequent fertility amongst women who ultimately developed premature ovarian failure, 22% had a child after treatment, compared to 41% who did not later develop premature ovarian failure.
There is some evidence that non-alkylating chemotherapy carries only a small additional risk of POI. Patients treated with pelvic radiotherapy and/or alkylating agents are clearly at increased risk. It is important in the counselling of these women to emphasize the impact on family planning, because although menstruation may return after chemotherapy the possibility of subsequent early menopause needs to be accounted for. This window of opportunity for fertility may also have implications for educational and career choices.
2.4
Mechanisms of Action of the Commonly Used Chemotherapy Drugs
Chemotherapy is the common term used to describe cytotoxic drugs, which generally act by selectively damaging or killing rapidly dividing cells. There are around 30–40 chemotherapeutic agents commonly employed in routine oncological practice (their development is well reviewed by Chabner and Roberts). Tumour cells are usually rapidly dividing and so these agents act to reduce the rate of tumour growth and/or reduce tumour size. A large number of normal host cells, including those of the ovary, are also in a state of cell division and these cells are also affected by cytotoxic therapy. Chemotherapy drugs can either be given as single agents or in combination. The rationale for the use of combination chemotherapy is that some agents have synergistic effects increasing efficacy, and, theoretically, agents with different mechanisms of action reduce the likelihood of cancer cell resistance developing. Of course, the use of agents that have some overlap of toxicity profiles increases the impact on non-tumour cells, including those of the reproductive system. Chemotherapy is usually administered in cycles whereby following a pulse of therapy, there is a period of days or a week before a subsequent pulse is administered. This is in order to allow essential normal cells (such as those of the bone marrow and the gastrointestinal tract) to recover, with the assumption that recovery in tumour cells is less efficient. These regimes are designed with anti-neoplastic effect as the priority, and generally revolve around the principle that as much chemotherapy as possible should be given within a set period of time without exposing the patients to an unacceptable risk in terms of other toxicities. In cancers where there is a clear relationship between dose and chance of cure, a myeloablative dose of chemotherapy can be given supported by a stem cell or bone marrow transplant. To date, little consideration has been given to the impact on female reproductive function in order to achieve these oncological goals.
The mechanisms of action of the major categories of chemotherapy drugs are described below. Examples of agents that fall into each category are shown in Table 2.1 .
| Alkylating Agents | Antimetabolites | Antitumour Antibiotics | Mitotic Inhibitors | Platinum Drugs | Topoisomerase Inhibitors |
|---|---|---|---|---|---|
| ALKYL SULFONATES | Capecitabine | ANTHRACYCLINES * | EPOTHILONES | Carboplatin | TOPO 1 INHIBITORS |
| Busulfan | Cladribine | Daunorubicin | Ixabepilone | Cisplatin | Irinotecan |
| ETHYLENIMINES | Clofarabine | Doxorubicin | TAXANES | Oxaliplatin | Topotecan |
| Altretamine | Cytarabine | Epirubicin | Docetaxel | TOPO 2 INHIBITORS | |
| Thiotepa | Floxuridine | Idarubicin | Paclitaxel | Etoposide | |
| NITROGEN MUSTARDS | Fludarabine | Mitoxantrone | VINCA ALKALOIDS | Teniposide | |
| Chlorambucil | 5-Fluorouracil | OTHERS | Vinblastine | ||
| Cyclophosphamide | Gemcitabine | Actinomycin D | Vincristine | ||
| Ifosfamide | Hydroxyurea | Bleomycin | Vinorelbine | ||
| Mechlorethamine | 6-Mercaptopurine | Mitomycin C | OTHERS | ||
| Melphalan | Methotrexate | Trabectedin | Estramustine | ||
| NITROSOUREAS | Pemetrexed | ||||
| Carmustine (BCNU) | Pentostatin | ||||
| Lomustine | Thioguanine | ||||
| Streptozocin | |||||
| HYDRAZINES/TRIAZINES | |||||
| Dacarbazine (DTIC) | |||||
| Procarbazine | |||||
| Temozolomide |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree