Keywords
apoptosis, chemotherapy, fertility, ovary, preservation, primordial follicles
9.1
Introduction
Recent advances in our understanding of the mechanisms underlying the impact of cytotoxic drugs on the ovary have opened up new directions for the protection of ovarian function from chemotherapy-induced damage. Studies are providing greater detail as to the pathways and factors triggered within the different cell types of the ovary by each drug class. As we gain increased knowledge of the specifics of these pathways, we reveal new targets for protective agents to reduce or prevent ovarian damage.
Most preclinical research on protective agents has concentrated on drug groups known clinically to have severe effects on follicle reserve, such as alkylating agents (cyclophosphamide, busulfan and dacarbazine), platinum complexes (cisplatin, carboplatin) and taxanes (paclitaxel). A large number of studies have also examined the anthracyclin antibiotic doxorubicin (DXR), which has been shown to affect the follicle reserve experimentally but which is currently considered of low clinical risk for premature ovarian insufficiency (POI). Alkylating agents and platinum complexes work in a similar fashion, creating DNA crosslinks, which, in turn, cause DNA breaks, ultimately triggering apoptosis. The taxanes are microtubule-stabilizing agents, as distinct from DNA-damaging drugs, but it has been demonstrated that paclitaxel acts via Bax to induce apoptosis. DXR is an intercalating agent that blocks DNA replication and causes double-stranded (ds)DNA breaks; it induces apoptosis primarily in the stroma and granulosa cells of growing follicles. Within the oocyte, anthracyclin agents such as DXR have been shown to induce chromosomal fragmentation as well as fragmentation of the cytoplasm into apoptotic bodies.
9.2
Ovarian Protection by Affecting Apoptotic Pathways
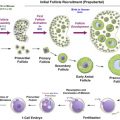
The most extensively researched pathway in the context of chemotherapy- and radiotherapy-induced ovarian injury is the apoptotic pathway leading to cell death in response to DNA damage. Cytotoxic drug-induced apoptosis has been most clearly demonstrated in growing follicles ( Figure 9.1B ), and has been shown to originate in the proliferating granulosa cells. Within the mature oocyte, apoptosis is mediated by several molecules including ceramide, Bax, and the caspases. Ceramide has been identified as an initiator of DXR-induced apoptosis in oocytes, and Bax, a protein produced by the Bcl2-gene family, plays an essential role in DXR-initiated apoptosis in mature mouse oocytes. Caspases, members of the CASP protease family, have been universally shown to play a pivotal role as cell death effector molecules, and are central in the apoptotic pathway of mature oocytes. Caspases-2, -12 and -3 specifically, have been shown to play a role in apoptosis of mature oocytes. Mouse oocytes pretreated with a caspase inhibitor as well as mature oocytes derived from mice that lack expression of caspase-2 show a marked resistance to DXR-induced apoptosis.
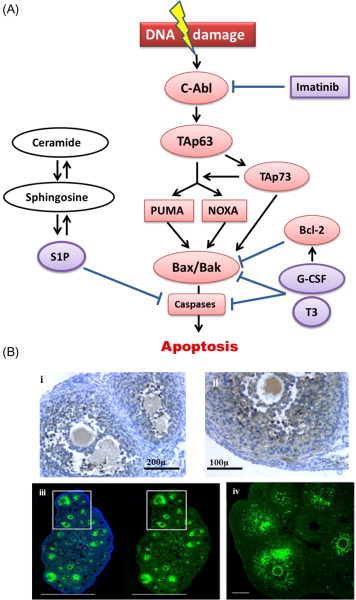
Investigation of apoptotic pathways within the dormant primordial follicle have revealed that p63, a homologue of anti-oncogene p53, found in the nucleus of oocytes, and specifically the p63 isoform TAp63, is a key mediator of the DNA damage apoptosis pathway in the response of primordial follicle oocytes to DNA injury. The p63 pathway is upregulated when oocytes are exposed to external triggers of DNA damage such as radiation and chemotherapy drugs such as cisplatin, and loss of p63 in mouse oocytes results in resistance to the apoptotic effects of radiation and cisplatin. Tap63 activates apoptosis via proteins BAX (Bcl-2-associated X protein) and BAK (Bcl-2 homologous antagonist killer). The activation of apoptosis is mediated by TAp73, either directly or through the activation of p53 upregulated modulator of apoptosis (PUMA) and phorbol-12-myristate-13-acetate-induced protein 1 (NOXA). Oocyte-specific deletion of PUMA and/or both PUMA and NOXA in mice prevents γ-irradiation-induced apoptosis and can produce healthy offspring, indicating that the protected oocytes are capable of DNA repair and subsequent normal function. Figure 9.1 schematically summarizes the key factors in the relevant apoptotic pathways.
Each of these factors in the apoptotic cascade represents a potential target for blocking the effects of cytotoxic treatments on the ovary. One important concern with developing any agent that interferes with the apoptotic pathway as a potential protectant is that, since induction of apoptosis is a key anticancer action of many of the chemotherapy drug classes, interference with the apoptotic pathway may inhibit the anticancer effects of these drugs. A further concern is that blocking apoptosis in DNA-damaged oocytes could allow survival of germ cells that are beyond the repair capabilities of the DNA repair molecules. Fertilization of these genetically compromised oocytes could lead to an increased risk of miscarriage, fetal death or malformation.
9.2.1
Imatinib
c-Abl protein tyrosine kinase has been shown to act as a “switch” for TAp63 transcriptional activity and the apoptotic pathway following exposure to the chemotherapy agents cisplatin and DXR. Other studies have further demonstrated that c-Abl plays a role in the maintenance of genomic integrity by dealing with DNA breaks in both meiotic and mitotic cells. Imatinib is a competitive tyrosine kinase inhibitor clinically used in the treatment of cancer, most notably chronic myelogenous leukaemia. It was investigated as an agent to prevent primordial follicle loss caused by cisplatin based on its role as a c-Abl kinase inhibitor. Results from that study demonstrated that co-administration of imatinib with cisplatin in mice reduced primordial follicle loss as well as improving fertility and reproductive outcomes. However, a subsequent study contested these results, finding that imatinib neither protected primordial follicle oocytes from cisplatin-induced apoptosis nor prevented loss of fertility in two independent strains of mice. The conflicting results are likely due to a number of key differences in study design, but the role of c-Abl in the induction of oocyte degeneration has since been supported using another c-Abl inhibitor, GNF-2, while others have confirmed the protective effect of imatinib. An in vitro study demonstrated that imatinib reduced the adverse effect of cisplatin on follicle health in cultured ovaries, while Kim et al. showed that imatinib inhibited the cisplatin-induced upregulation of apoptotic mediators and reduced the damage to primordial follicles in a novel system of in vitro organ culture followed by subrenal grafting that enabled assessment of longer term effects.
Additional study is needed both to ascertain whether imatinib interferes with the anticancer action of cisplatin, since c-Abl has been shown to mediate cisplatin’s action in a number of cancer cell lines, and also to assess the genetic integrity of oocytes rescued from apoptosis.
9.2.2
Sphingosine-1-Phosphate
Sphingomyelin hydrolysis is a ceramide-promoted trigger of apoptotis and sphingosine-1-phosphate (S1P) is an inhibitor of this particular apoptotic pathway. In vivo treatment of human ovarian tissue xenografts in mice with S1P increased vascular density and angiogenesis, and reduced follicle apoptosis. In vivo administration of S1P before radiation exposure resulted in a dose-dependent preservation of follicle numbers in mice, and a virtually complete preservation of both primordial and growing follicles when S1P was administered at high doses. In similar studies, S1P pretreatment was shown to reduce irradiation-induced primordial follicle depletion in rats, primates, and xenografted human ovarian tissue.
S1P pretreatment demonstrated a protective effect in mice treated with dacarbazine, reducing follicle loss and increasing pregnancy rates. Pretreatment and ongoing administration of S1P to mice carrying xenografted human ovarian tissue prevented the significant apoptosis of follicles caused by both cyclophosphamide (Cy) and DXR treatment. Ex vivo , S1P treatment of mouse oocytes conferred resistance to DXR-induced apoptosis. Results have not been uniformly positive, however, with one study reporting no reduction in Cy-induced follicle loss in S1P-treated rats.
One limitation of S1P is that, because of its very short plasma half-life, it cannot be administered by systemic injection, and would either require continuous administration (studies used mini-osmotic pumps) or injections directly into the ovary. Local administration would, in theory, reduce the possibility that S1P would interfere with the therapeutic effects of chemotherapy drugs. Studies that have examined offspring derived from female mice and macaques that received S1P treatment prior to radiation showed no significant abnormalities of any kind, possibly allaying concerns regarding persistent DNA damage in rescued oocytes, but no equivalent studies have been conducted after chemotherapy treatment.
9.2.3
Granulocyte Colony-Stimulating Factor
Granulocyte colony-stimulating factor (G-CSF) has been shown to significantly reduce the destruction of primordial follicles caused by Cy, busulfan and cisplatin, as well as preventing damage to the microvessels and reducing markers of DNA damage in oocytes of early-growing follicles to control levels. An extended mating study further demonstrated that mice that received G-CSF at the time of treatment produced significantly more pups per litter than those that received chemotherapy only. The mechanism of action of G-CSF requires clarification. It is possible that the protective effects of G-CSF on blood vessels decreases the chemotherapy-related blood vessel loss and the associated focal ischaemia shown by Meirow et al. to be a contributing cause of follicle loss. However, the strongest hypothesis suggested by the authors is based on the anti-apoptotic effects of G-CSF. G-CSF has been shown to induce the expression of Bcl-2 and decrease Bax translocation, resulting in inhibition of apoptosis.
While confirmation of these data and further examination of the mechanism behind the protective effect of G-CSF are still warranted, it claims certain advantages over some proposed attenuating agents as it is currently in clinical use in cancer patients for prevention of chemotherapy-induced neutropenia and has been shown not to reduce the efficacy of chemotherapeutic agents.
9.2.4
Thyroid Hormone (T 3 )
Thyroid hormone, T 3 , has been shown to have anti-apoptotic effects in vitro in a number of different cell lines, including ovarian somatic and granulosa cells. One study showed that T 3 reduced apoptosis in granulosa cells exposed in vitro to paclitaxel by downregulating caspase 3 and BAX ; however, another study showed no protective effect of T 3 when administered in vitro to mouse ovaries with docetaxol.
9.2.5
Tamoxifen
Tamoxifen is an oestrogen receptor antagonist used in adjuvant treatment of hormone-sensitive cancers. Two rodent studies have proposed a protective effect when tamoxifen is given during chemo/radiotherapy. Ting et al. showed that in vivo treatment with tamoxifen significantly reduced follicle loss caused by Cy, as well as improving fertility outcomes post-treatment. In addition, the study demonstrated that while in vitro DXR exposure increased oocyte fragmentation, the effect was reversed with tamoxifen. There are data to suggest that tamoxifen in conjunction with chemotherapy increases patient risk of long-term amenorrhoea; however, in the absence of chemotherapy, there was no association between use of tamoxifen and onset of long-term amenorrhoea. Furthermore, since the effects of tamoxifen on the ovary are complicated and not well understood, amenorrhoea while on tamoxifen does not, in itself, indicate menopause. The mechanism of a possible protective effect of tamoxifen against chemotherapy has not been investigated and is thought to relate either to its role as an oestrogen agonist with associated anti-apoptotic and anti-oxidant effects, or possibly its effects on the pituitary–gonadal axis. Similarly, while whole body irradiation in rats significantly reduced primordial follicle numbers and anti-Müllerian hormone (AMH) serum levels, and increased oxidative stress markers, co-treatment with tamoxifen ameliorated all of these effects, as well as rescuing fertility post-treatment. That study demonstrated that tamoxifen treatments resulted in a significant increase in both transcription and translation of insulin-like growth factor 1 (IGF-1), which has been shown to modulate gonadotropin action in the ovary by augmenting granulosa cell follicle-stimulating hormone (FSH) receptor expression and potentiating FSH action, as well as to exert anti-oxidant and cryoprotective effects.
It is worth noting here that it is difficult to explain how any effects on the pituitary–gonadal axis would protect the ovarian follicle reserve, since gonadotropins act only on the growing follicles, and primordial follicles are not directly under gonadotropin influence. This is also discussed in Chapter 8 in relation to the potential protective role of GnRH agonist treatment.
9.3
PI3K Follicle Activation Pathway and Ovary Protection
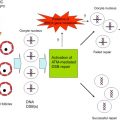
The most recent theory of chemotherapy-induced destruction of dormant follicles suggests that in vivo , chemotherapy agents such as Cy and cisplatin trigger an initiation of dormant follicle growth immediately following chemotherapy exposure, occurring simultaneously with large follicle apoptosis ( Figure 9.2 ). The activation of dormant follicles was shown to be mediated by an upregulation in the PI3K/PTEN/Akt signalling pathway, whose role in follicle quiescence has been well established by numerous knock-out mouse models as well as its relevance to the human follicle by in vitro studies on cortical tissue.
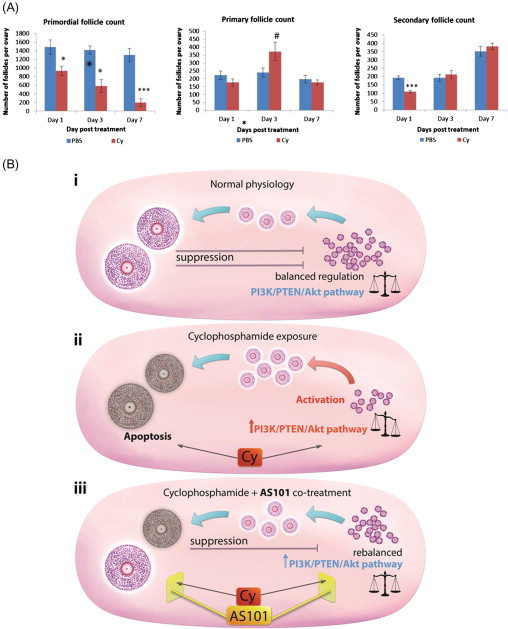
The route by which chemotherapy terminates follicle dormancy and induces activation of the PI3K/PTEN/Akt pathway may be via direct influence on the oocytes and pregranulosa cells of primordial follicles, or indirectly via chemotherapy-induced destruction of larger follicles. Destruction of large follicles reduces negative regulation on the primordial follicle pool, thereby resulting in activation of primordial follicles in an attempt to replace the dying cohort of growing follicles. This has been suggested as the mechanism behind primordial follicle activation and depletion seen following exposure to carcinogen and ovotoxicant 3-methylcholanthrene.
9.3.1
AS101
The immunomodulator AS101 is a non-toxic, tellurium-based compound that acts on the PI3K/PTEN/Akt pathway. In vivo treatment of mice with AS101 reduced Cy-induced loss of primordial follicles as well as reducing apoptosis in granulosa cells of growing follicles and improving reproductive outcomes, via its modulation of the PI3K/PTEN/Akt pathway. This mechanism could be by either direct protection or reduced activation of primordial follicles, or by a combination of both. No increase in fetal malformations was observed in mice whose fertility was protected by AS101 treatment, indicating that the functionality and genetic integrity of these rescued oocytes were not compromised. AS101 has been shown not to interfere with the primary anti-neoplastic activity of Cy in vivo , or with Cy metabolites in vitro .
9.4
Other Potential Methods of Reducing Chemotherapy-Induced Ovotoxicity
9.4.1
Interference with Transport: Bortezomib
DXR has been shown to accumulate first in stromal cells, and progressively move towards the follicles, so that DXR-induced dsDNA breaks occur first in stromal cells (within 2 hours of administration), followed by the granulosa cells (within 4 hours) that surround the oocytes. In order to affect DNA damage, DXR must be transported across the nuclear membrane, via binding to proteasomes. One study investigated the use of the high-affinity proteasome inhibitor bortezomib, which directly competes with DXR for binding to the proteasome, thus preventing DXR nuclear accumulation. By decreasing DXR accumulation within ovaries, bortezomib pretreatment prevented DXR-induced DNA damage and the activation of downstream apoptotic pathways in all ovarian cell types, and resulted in improved reproductive outcomes in a rodent model. An inherent advantage of bortezomib is that it is already in clinical use as an anticancer agent.
9.4.2
Upregulation of Multidrug Resistance Gene ( MDR1 )
For drugs whose main mechanism of action is via the granulosa cells, another possibility for protection lies in the manipulation of phase III drug transporter enzymes such as MDR1. MDR1 is involved in the metabolism, elimination and detoxification of chemotherapy. Upregulation of MDR1 has been linked to chemoresistance by promoting the transport and efflux of various lipid-soluble anticancer agents. Upregulation of MDR1 using retroviral transduction in a granulosa cell line was shown to protect cells from the toxic effects of DXR and paclitaxel in a dose-dependent manner. MDR1 -transduced granulosa cells showed significantly increased cell survival following treatment with either DXR or paclitaxel. Conversely, in a different study, inhibition of MDR transporters in mouse and human oocytes led to increased susceptibility to cyclophosphamide toxicity.
A particular concern with this method of chemoprotection is that it utilizes retrovirus therapy, raising the possibility of viral gene transfer to germ cells and ultimately to potential fetuses.
9.4.3
Drug Encapsulation
Another direction that has been investigated to reduce the toxic side effects of chemotherapy, particularly for solid tumours, is drug encapsulation with/without tumour targeting. One successful example of this method is a pegylated (polyethylene glycol-coated) liposome-encapsulated form of DXR, which reduces the cardiotoxicity of the drug and is currently in clinical use for the treatment of ovarian cancer and multiple myeloma. A recent study examined the possibility that nano-encapsulation of arsenic trioxide (As 2 O 3 ; used in the treatment of haematological malignancies) may increase its efficacy against solid tumours while simultaneously reducing its effects on the ovary. The study demonstrated that nano-encapsulation of As 2 O 3 resulted in lower peak plasma levels, limited its tissue distribution and reduced its impact on ovarian and follicular function. While the study did not examine the impact of the encapsulated drug on ovarian reserve or function following treatment, the concept holds great potential and warrants additional investigation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree