Keywords
anti-Müllerian hormone, antral follicle count, chemotherapy, infertility, ovarian reserve, premature ovarian insufficiency
3.1
Introduction
Assessment of ovarian toxicity in the clinical setting is important in determining the effects of exposures on individual women and to compare different exposures in groups of women, for example those on different chemotherapy regimens. Such knowledge can also be used prospectively to inform discussion about likely effects on future fertility, for example in patients facing cancer therapies or other treatments involving cytotoxic agents, e.g., in rheumatic conditions such as systemic lupus erythematosus. This approach is also potentially applicable to prepubertal girls; up until now, however, this has proved very difficult in the absence of being able to assess the prepubertal ovary, although that is now changing as will be described below.
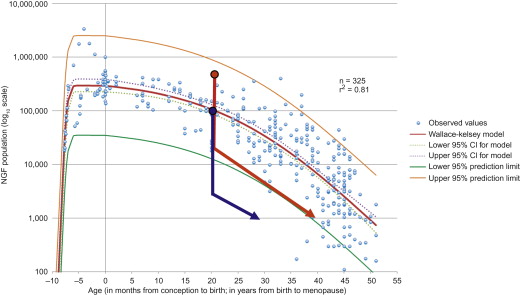
The essential basis of assessment is that the human ovary contains a finite pool of primordial follicles, which are formed in utero . A small number of follicles is activated daily into the growth phase, proceeding through preantral and subsequently the antral stages of development, becoming increasing dependent on follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the pituitary. While most follicles are destined to become atretic, increasing selection results in a single follicle being selected to become dominant each menstrual cycle to become the ovulatory follicle. While numerically the smallest stages of follicle are by far the most abundant in the ovary, it is at the later antral and preovulatory stages that the follicle is most endocrinologically active, which is necessary for preparation for potential pregnancy. While a constant supply of small preantral follicles is consequently required to support the selection process that ultimately leads to ovulation, the number of small follicles declines throughout a woman’s reproductive life, whereas she continues to produce a single ovulatory follicle until the menopause. This underpins the absence of a clear relationship between ovulatory activity, i.e., oestrogen production, and the ovarian reserve.
3.2
The Use of Chemotherapy-Related Amenorrhoea
The presence of cyclic menstruation, itself reflecting oestrogen production by the large antral and preovulatory follicle population, is the most readily apparent index of ovarian activity, with its absence indicating the loss of ovarian function. Chemotherapy-related amenorrhoea (CRA) is the most widely used index of ovarian toxicity in cancer studies and it undoubtedly remains a useful measure of assessment. It is generally used to reflect premature ovarian insufficiency (POI), previously termed “premature menopause” or “premature ovarian failure”. It is, therefore, taken to indicate the absence of follicular growth in the ovary and, by implication, depletion of the primordial pool, i.e., the ovarian reserve. While it should be noted that amenorrhoea can also result from a lack of gonadotropins, which may occur after, for example, cranial irradiation, in the great majority of instances the toxicity will clearly be to the ovary and the interpretation that it does indeed indicate POI is thus fairly robust. The precise definition does, however, vary significantly between studies (e.g., how many months of amenorrhoea are required). It is also important to recognize in this context that the amenorrhoea results from lack of oestrogen stimulation of the endometrium. As growing (oestrogen-producing) follicles are generally sensitive to chemotherapy, the acute toxicity to these follicles can result in CRA independent of any effect on the primordial pool. Oestrogen is, therefore, not a useful biomarker of damage to the primordial and early growing pool of follicles, with the clinical scenario being the frequent occurrence of amenorrhoea in women receiving chemotherapy who later recover and resume normal menstrual cycles – thus indicating that at least some of the ovarian reserve remains.
Many retrospective analyses have compared different chemotherapy regimens on the prevalence of amenorrhoea, as have large prospective analyses. A study by Petrek and colleagues is a very clear example of the acute impact of chemotherapy in women with breast cancer, with many developing CRA during and following treatment, with recovery of menstruation in some women following the end of treatment. The overall prevalence of ongoing menses, however, subsequently declines as more women develop POI. This study also very strikingly shows the huge impact of the woman’s age on the likelihood of ongoing menses after chemotherapy, varying from approximately 90% in women aged less than 35, to about 30% in women aged 40 and above. An important addition to these analyses is the demonstration that women who retain ovarian function after chemotherapy are at increased risk of an early menopause, indicating the consequence of loss of much but not all of the ovarian reserve during chemotherapy.
3.3
Biomarkers of the Ovarian Reserve
There has long been interest in the development of accurate tools to detect the progressive loss of the ovarian reserve, which occurs with ageing, and indeed the incomplete loss from ovarian toxicity. This is in many ways analogous to the situation in assisted conception, where there is a need to distinguish between women with varying likely responses, from those with a poor or minimal response to superovulation, to those who will show an excessive response. There is a very substantial literature on assessment of the ovarian reserve in this context, with initial studies exploring the value of FSH measurement, either basally or after a stimulatory test. FSH is clearly of value in diagnosing the menopause or POI, but it is of lesser value in detecting women of a low ovarian reserve. This is to be expected given its physiological role in selection of the dominant follicle and the later stages of antral follicle growth, thus being central to the ovulatory function of the ovary rather than the regulation of the early stages of follicle growth.
Current interest focuses around the use of measurement of circulating anti-Müllerian hormone (AMH), and ultrasound assessment of the number of small (generally 2–10 mm diameter) antral follicles: the antral follicle count (AFC). In the context of assisted reproduction, both are of comparable value in identifying both poor responders and over-responders. The term “ovarian reserve” is generally used in assisted reproduction to indicate the response to ovarian stimulation, although it is important to recognize that the follicles that will be recruited by exogenous FSH are already at a relatively advanced stage of growth, i.e., at least at early antral stages. This analysis, therefore, does not directly reflect the true ovarian reserve, i.e., the primordial pool, although in adult women in normal healthy circumstances there is a relationship between the two. Different relationships hold, however, in childhood and adolescence, where AMH rises despite a falling ovarian reserve, although individual girls retain relative AMH levels across that time period. AMH can also be influenced by external factors, most importantly in this context health status, with reductions in AMH in proportion to markers of general ill-health, including pyrexia and anaemia, and during administration of hormonal contraception.
AMH is produced by the granulosa cells of growing follicles, with expression initiated as soon as the follicle starts to grow, but it is not produced by the non-growing pool. Expression continues through to the antral stages, but shows a rapid decline at a follicle diameter of about 8–10 mm diameter, i.e., at the time the follicle is potentially selected for dominance. There is very little AMH production by larger antral follicles beyond this stage, which is of very substantial clinical benefit as it means that there is very little variation within the menstrual cycle. Thus, blood sampling can be taken at any time, in contrast to the measurement of FSH, which needs to be taken in the early follicular phase. Most of the AMH in the circulation is thought to come from the pool of small antral follicles detected by ultrasound, and there is generally a very close relationship between AMH and AFC measurements. Their value in assisted reproduction is strengthened by the fact that it is these same follicles that will be stimulated by exogenous FSH. At present, there remains no direct way of assessing the primordial follicle pool without using destructive histological analysis, although this remains an ideal. The limited activity of primordial follicles is a major hurdle in the development of such a biomarker. AMH and AFC have both been used to assess ovarian toxicity of chemotherapy regimens and it is clear that they are of value in this regard. It is, however, very important to recognize that what they are and are not measuring.
3.4
AMH and Determination of Gonadotoxicity
3.4.1
After Chemotherapy
AMH was first shown to be of potential value in assessing ovarian toxicity in cancer patients in a study of young adult women with regular menstrual cycles who were survivors of childhood cancers. AMH but not FSH or inhibin B was significantly reduced in cancer survivors compared to age-matched controls. Ultrasound was also used in that study, and showed a reduced ovarian volume in cancer survivors having spontaneous cycles, although AFC was not reduced. Several studies have subsequently confirmed reduced AMH in survivors of adult and childhood cancer, with some of these finding clear relationships between the degree of loss of AMH and the gonadotoxicity of the treatment involved. Thus, women who have received alkylating agent-based therapy have lower AMH levels than those who received less toxic therapy : this has also been confirmed in prospective studies. A dose response is also apparent, with progressively lower AMH concentrations in women treated with more cycles of alkylating-based therapy for lymphoma. Similarly, abdominopelvic irradiation and stem cell transplantation have very marked effects on AMH levels. AMH has also been used to show the marked gonadotoxicity of radiotherapy involving the ovaries. In general, comparable data have been obtained using AFC, although the literature on this is substantially sparser.
3.4.2
Prospective Analyses
There are now a growing number of studies that have prospectively assessed AMH as a marker of gonadotoxicity, thus making it possible to use pretreatment samples to show the fall in AMH in individuals, rather than requiring age-matched controls. This has confirmed the rapid loss of AMH during chemotherapy, reflecting the acute loss of growing follicles. The fall in AMH appears more rapid and complete than changes in oestradiol and inhibin B, at least with the chemotherapy regimens (for early breast cancer) in that study. Further studies are required to dissect how useful this might be to reflect toxicity of different regimes on different stages of follicle growth. Prospective studies can also investigate the potential recovery of ovarian function after chemotherapy, and this has been clearly demonstrated to reveal differences between different therapeutic regimens for lymphoma. Women with higher pretreatment AMH also were found to have more rapid recovery than those with lower pretreatment AMH.
It is now becoming clear that pretreatment AMH concentrations can be used to predict the likelihood of long-term ovarian function after chemotherapy. This is in keeping with the concept that women with a higher pretreatment ovarian reserve are more likely to have more follicles that will survive treatment and that they will, therefore, have sufficient ovarian reserve to support clinical ovarian activity, i.e., continuation of or resumption of menses ( Figure 3.1 ). This has been most clearly shown thus far in women with breast cancer, and data are required to confirm its relevance in other conditions, with their different associated treatment regimens and age profile of patients. While AMH appears of particular value in this context, it is important to recognize that age also has a role in this pretreatment assessment, as it remains an independent predictor. This is analogous to data emerging on the interaction between age and AMH in predicting the menopause in normal women. Ovarian function in these studies is of value in showing the endocrine activity of the ovary, but there are no equivalent data in relation to fertility. This is of significant importance as surveys of women treated for cancer, either as adults or in childhood, show a high prevalence of subfertility in those with ongoing ovarian activity ; the cause of this is unclear.