(1)
State University of New York at Buffalo Kaleida Health, Buffalo, NY, USA
Rationale
The rationale for intra-arterial infusion chemotherapy is fairly simple: The first pass of the drug in high concentration occurs through the capillary network of the tumor before mixing with the circulating blood volume. An obvious requirement for the use of intra-arterial chemotherapy is that the tumor bulk is concentrated in an area supplied preferably by a single identifiable artery, though regional chemotherapy can also be applied without major difficulty if the tumor is supplied by two arteries.
Drug Concentration and Distribution in the Regional Tissues
Infusion chemotherapy has not been studied fully. Experimentally, one can show that significantly higher drug concentrations can be obtained in liver tissue when the drug is infused through the hepatic artery, as compared with intravenous (IV) infusion of the same amount of the drug. After intra-arterial infusion, however, the tissue levels of the drug, though higher than those obtained with systemic IV administration, are more variable and heterogeneous than the homogeneous tissue levels of the drug following systemic IV administration [1]. The obvious reason for this problem is that as the drug is administered slowly into the hepatic artery, it forms a thin stream within the lumen of the vessel, surrounded by the rushing blood flow. Its failure to mix freely with the surrounding blood can cause the levels obtained in the regional tissues to be quite variable. When peripheral IV administration is used, the drug goes through the right side of the heart, the pulmonary circulation, and the left side of the heart before entering the systemic circulation. The drug becomes thoroughly mixed with the blood while going twice through the heart chambers, with their pulsatile action, so the levels obtained are quite homogeneous. Theoretically, the problem of inadequate mixing of the drug into the blood as it enters an artery may be ameliorated by using a pulsatile flow for the injected drug solution, but this idea has not been tested. Another problem occurring with intra-arterial infusion is that only a small percentage of the drug passing through the cannulated artery is diffusing through the capillaries into the regional tissues; the bulk of the drug is transferred rapidly through the capillary network of the regional tissues into the systemic circulation.
Theoretically, a way to avoid this problem and to enhance the regional tissue levels of the drug would be temporary occlusion of the artery proximal to the tip of the catheter, with rapid drug infusion over a period no longer than the time that the arterial occlusion can be tolerated. Simple interruption of the arterial flow proximal to the site of intra-arterial injection, without hemodynamic alterations on the venous side of the regional circulation, does not significantly increase the tissue levels of infused drugs in a canine model or cause the clinical changes in the skin characteristic of a successful perfusion (redness, blistering, ulcerations – although clinically less overt changes are aimed for). Occlusion only of the common femoral vein is not effective. Simultaneous complete occlusion of the venous outflow when that is anatomically feasible (e.g. with the application of a tourniquet at the root of an extremity) dramatically increases the drug concentration in the regional tissues and causes all the changes of a successful perfusion with very low drug doses [2] and only partial occlusion of the artery (infusion at mean arterial pressure). The advantages of the Tourniquet Infusion (TI) over perfusion are that it does not require an operation, it can be repeated every 2–3 weeks for several cycles, and it does not carry the risk of systemic toxicity [3]. Some tumors undergo complete regression, such as all three of our cases of diffuse Kaposi sarcoma of the lower extremity below the knee [4]. These patients were treated with doxorubicin (20 mg in 100 mL of normal saline per course) with the TI method for two courses. Of 24 evaluable patients with extremity in-transit lesions from melanoma treated with the TI method, the overall objective response rate was 75 %. A complete response was seen in 29 % of patients (including one patient with failure to respond to previous isolation hyperthermic perfusion elsewhere), >50 % response was seen in 46 % of patients, <50 % response was seen in 12.5 % of patients, and disease progressed in 12.5 % of patients [5]. However, the TI method results in inhomogeneous skin changes, indicating uneven drug distribution. This problem should be corrected and the optimal drug doses and specific drugs worked out for different extremity neoplasms before this method is accepted for wide use.
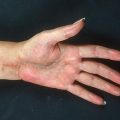
Skin changes are observed up to where the lower edge of the tourniquet cuff had been placed (Fig. 24.1). They heal slowly over several weeks (Fig. 24.2). They require no special care, are left mostly exposed to the air, and treated on an outpatient basis. In a small series of patients with extremity sarcoma the patients did well with preoperative TI followed by resection (Figs. 24.3, 24.4, 24.5, and 24.6). TI was administered with normothermia. However, the TI method results in inhomogeneous skin changes indicating an uneven drug distribution (Fig. 24.7). The effects of TI chemotherapy on the treated leg can be restricted for a tumor at the knee area by using two tourniquets, one above the tip of the intraarterial catheter and the other below the distal extent of the tumor (Fig. 24.8).
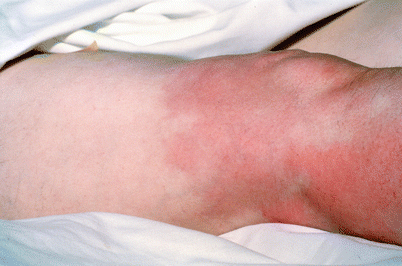
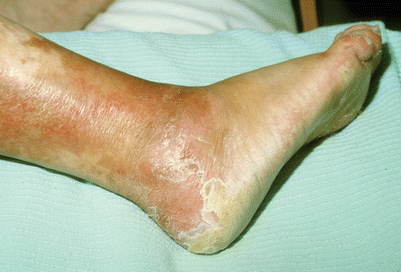
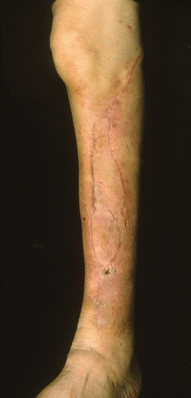
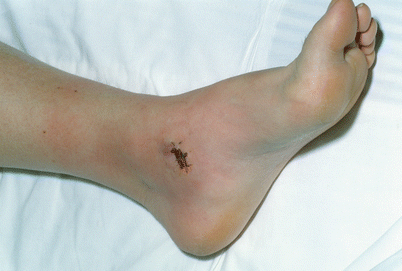
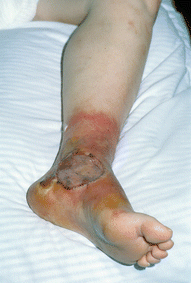
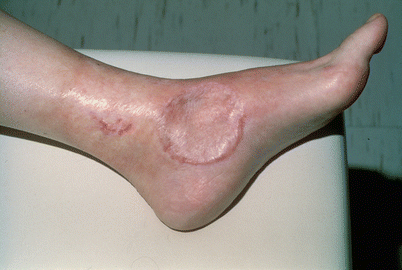
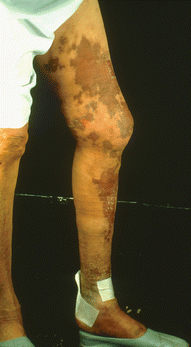
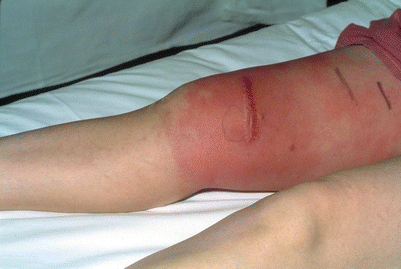

Fig. 24.1
Erythema of the leg to the lower thigh where the tourniquet had been placed during infusion

Fig. 24.2
Post-infusion changes in the right leg. Kaposi’s lesions have regressed

Fig. 24.3
Post-infusion changes in the left leg several months after TI combined with surgical resection of three sites of recurrent liposarcoma (after repeat surgery and radiation elsewhere). Twenty years later he was well

Fig. 24.4
Site of biopsy of sarcoma in the left medial ankle. Erythema at that site following TI. The skin changes were localized in that area by having the tip of intraarterial catheter in the popliteal artery and applying warm compresses in the left ankle

Fig. 24.5
The patient of Fig. 24.4 was operated a few hours after TI (local excision and skin grafting). Picture taken a few days later

Fig. 24.6
Photograph of the same patient as in Fig. 24.5, taken several months later. The patient remained well in the next 10 years

Fig. 24.7
Skin changes in the leg of a patient with multiple in-transit melanoma lesions. Observe the lack of uniformity in the distribution of these skin changes indicating non-uniform distribution of the infused drug during TI. After several courses of TI therapy the in-transit lesions regressed

Fig. 24.8
A patient with sarcoma in the distal thigh above the knee. The drug distribution during the TI is restricted to that area by using a tourniquet above and another one below the knee during drug infusion. TI is complemented with resection of the tumor
In a patient with osteogenic sarcoma of the distal femur, three courses of TI using Doxorubicin and Cisplatin were given at 3-week intervals. A generous biopsy of the tumor area was then obtained, which showed negative for signs of tumor. Three more courses of TI were given, and then another biopsy was taken, which was also negative for signs of tumor. Three months later a repeat biopsy was negative, although 6 months afterwards, there was local recurrence of the tumor accompanied by pulmonary metastases. Apparently, although areas at the tumor site with repeat open biopsy after TI were negative, other microscopic areas, not accessed with the biopsy specimens, remained alive and gave rise to the recurrence. Subsequently, even when a primary extremity tumor undergoes clinical and/or histological regression with chemotherapy, it is not safe to rely exclusively on this effect, but rather to supplement it with a resection of this area.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree