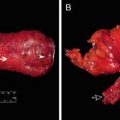
Fig. 15.1
Perineal defect exposing pelvic organs and creating significant dead space
Primary closure is the ideal choice for perineal defects . However, in many cases, this is not possible due to the size of the defect or other constraining factors, leading to primary wound closure complication rates of nearly 65% [2]. Hematoma, seroma, fistula formation, non-healing wound, and abdominal/perineal hernia are other frequent issues. These can lengthen hospital stays, cause significant morbidity, decrease mobility, and delay adjuvant therapy.
Because of potential issues with primary wound closure, many reconstructive surgeons rely on pedicled, vascularized soft tissue flaps for coverage of extensive perineal defects [3]. Vascularized soft tissue provides the nourishing effects of consistent blood flow such as increased oxygenation, delivery of growth factors, and higher cytokine concentrations. These flaps further assist wound healing by obliterating dead space and decreasing incisional tension.
In this chapter, we will provide a comprehensive overview of the reconstructive options for perineal defects (Fig. 15.2). With this information, the reader will understand the principles for the multidisciplinary management of post-resective perineal wound defects and prevention of associated complications, facilitating maximized patient outcomes despite the challenging nature of these cases.
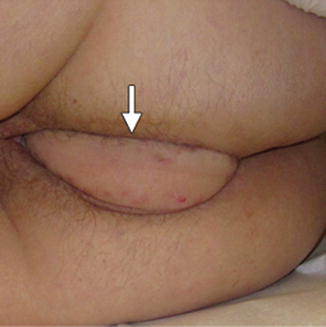

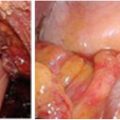
Fig. 15.2
Perineal reconstruction , with arrow indicating healing soft tissue reconstruction
Preoperative Assessment
The need for reconstruction must be balanced with the physiological state of the patient . Overall determination of the patient’s health is key to planning the operative repair of a perineal defect; a patient too sick for surgery will not benefit from even the best reconstructive effort. For those patients healthy enough for surgery, accurate assessment of the surgical site should be performed, perhaps under anesthesia if necessary, as in the case of prior failed primary closure. The long term prognosis of the patient is also a contributor to this equation. Reconstruction will have tremendous positive impacts on quality of life so it may be considered in palliative cases too.
Medical Comorbidities
Preoperative risk stratification by pre-existing health conditions or environmental factors (e.g., smoking) must be undertaken to understand patient-specific relative risks for wound complications. One major element that must be addressed is smoking. The effects of smoking on the microcirculation can severely compromise soft tissue healing, and the patient should cease smoking at least 4 weeks before surgery [4]. Nutrition is another modifiable factor that must be optimized prior to undertaking major reconstructive surgery [5, 6]. Ideally, pre- and postoperative enteral feeding with supplemented protein will be ordered.
Radiation Therapy
Many rectal cancer patients will be treated with pelvic radiation prior to tumor resection and reconstruction. The exposure of the perineum to radiation can be problematic to the reconstructive effort. Radiation therapy injures healthy tissue which will not be resected, especially in the microcirculation of the defect and the surrounding areas. This effect produces decreased perfusion and diminished wound healing capacity [3]. Knowledge of the timing, dosage, and location of prior or planned radiation therapy is crucial to the reconstructive surgeon as he or she plans for debridement of irradiated tissue or recruitment of tissue outside the irradiated field for the reconstruction.
Chemotherapy
Neoadjuvant chemotherapy is often necessary in patients undergoing perineal reconstruction for oncological conditions. Similarly to radiation therapy, this treatment mainstay can compromise wound healing. Close, frank communication with the medical oncology team facilitates an accurate determination of if and when neoadjuvant chemotherapy is needed and how to address potential wound complications.
Imaging
Some cases may require imaging with computed tomography (CT) and/or magnetic resonance imaging (MRI). Radiographic studies are primarily used to understand the integrity of surrounding tissue and local vascular anatomy. Reconstructive surgeons can rely on these images to formulate more precise preoperative plans.
Timing of Reconstruction
Reconstruction of perineal defects should be done immediately, if possible. Rates of wound complications have been shown to be more favorable following immediate reconstruction in other anatomical locations [7]. The feasibility of immediate reconstruction is most frequently dictated by the status of the tumor and surgical excision margins. Other factors, such as advanced age, multiple comorbidities, or further need for adjuvant radiotherapy, will also influence the practicality of immediate reconstruction. Some instances will require delayed reconstruction, such as in cases of patient instability or extensive soft tissue defects. For these scenarios, negative pressure closure devices are the preferred intermediate treatment.
Reconstructive Surgical Tenets
The most fundamental goal of the reconstructive surgeon is to replace missing tissue with the most similar tissue possible—replace “like with like,” as the saying goes. Reconstructive efforts proceed from simple to complex schemes, as dictated by the nature of the wound. The functional aim of any reconstruction is to fill dead space and provide a tension-free closure.
Local flaps are a desirable option for surgeons to recapitulate form and function with similar tissue. Axial pattern flaps are the backbone of perineal reconstructions and are supplied by named blood vessels. They may be prepared as myocutaneous (muscle with skin), fasciocutaneous (deep muscle fascia with skin), or myofasciocutaneous (muscle, deep fascia, and skin) depending on the surgeon’s needs for restoring resected tissue.
Microvascular free tissue transfer is similar to local flaps in that the tissue used for reconstruction is supplied by a named blood vessel. The key difference is that this tissue originates from elsewhere in the body; in the process of the reconstruction, the native blood supply will be severed, and a new anastomosis will be created at the site of the defect, using the named recipient blood vessels and the flap’s donor vessels. Again, the nature of the defect will determine the specific origin and tissue composition (skin, adipose, fascia, muscle) of the donor flap. Microvascular free tissue transfer provides a handful of advantages, chief among them being the ability to recruit similar tissue from distant body sites, increasing the odds of a favorable functional and esthetic outcome. Another important advantage is as a source of viable, vascularized tissue for repair of previously irradiated or infected defects with compromised tissue. Limitations of this approach derive from donor-site morbidity and extended operation times. Pedicled soft tissue flaps are always the first line of therapy for the reconstruction of perineal wounds. Free flap microvascular transfer of soft tissue flaps to the perineum is a final option if all other local and pedicled options are exhausted.
Classification of Defect
As mentioned earlier, the most important aspect of reconstructive surgery is replacing “like with like”. Classifying a defect through identification of which structures are missing and/or compromised is the first step toward remediating the defect and recapitulating normal form and function. Perineal defects are organized according to the scheme presented in Table 15.1.
Table 15.1
Classification of perineal defects
Anatomic structure(s) involved | Missing tissue components |
|---|---|
Vaginal vault | S, MS, ST |
Vulvoperineal surface | MS, ST |
Scrotum | S, ST |
Penis | S, MS, ST |
Perineum and pelvic floor | S, ST |
Sacrum/bony pelvis | S, ST, ± osseous involvement |
For male patients with advanced rectal cancer, the penis, scrotum, and pelvic floor musculature are at risk of disruption during resection. Fortunately, total penile resection is rarely necessary. In those uncommon instances in which it is necessary, the goals of reconstruction are threefold: provide acceptable cosmesis, allow for normal urination, and facilitate normal sexual activity. Local flaps have been shown to be ineffective so free tissue approaches have been devised with greater success [9, 10]. Female patients have similar susceptibility to pelvic floor musculature injury along with potential need for vaginal vault reconstruction. The construction of a neovagina is aimed at providing a sexually functional vagina while closing the perineal wound and providing bulk filling to the pelvis to prevent bowel herniation. Many techniques have been used in the past with marginal success including skin-only grafts, intestinal grafts, and omental flaps [11]. The preferred approach is myocutaneous or fasciocutaneous flaps, such as vertical rectus abdominis myocutaneous (VRAM) flap or anterolateral thigh (ALT) flap, as discussed below [12].
Once a defect has been identified and appropriately classified, the quality of the tissue must be assessed. Viable vascularized tissue is essential for a successful reconstruction. Local flaps are the ideal, first-line reconstructive options. Potential donor sites will require adequate rotational lengths and soft tissue coverage of donor defects. Abdominal wall, thigh, or buttocks sites are frequently adequate donors. Should free tissue transfer be necessary, the patency of recipient vessels will need to be verified; failing this, appropriate planning for an arteriovenous loop should be undertaken. This can be performed using a saphenous vein graft anastomosed to femoral vessels or lumbar perforators.
Adjuncts to Flap Surgery
Negative Pressure Wound Therapy
In cases of delayed reconstruction, negative pressure wound therapy can bridge the time gap between tumor resection and reconstruction. When indicated, this treatment can enhance neovascularization and decrease edema, encourage granulation tissue formation, and stimulate circumferential wound contraction [13]. Beyond its use as a temporary measure in delayed reconstruction, negative pressure wound therapy facilitates healing by secondary intention in partial-thickness defects.
Tissue Expansion
Tissue expansion is a process by which local and regional tissues are slowly coaxed into controlled growth that will eventually enable them to be advanced into the wound and participate in the healing process. An inflatable prosthetic with a silicone shell is implanted at the time of tumor resection or during a second procedure; at subsequent office visits, serial saline injections are used to expand the implant and direct tissue growth. Once adequate growth has occurred, surgery is performed to definitively repair the defect with this expanded tissue. An unavoidable limitation of this method is that it cannot help in immediate reconstruction or in cases of exposed hollow viscous or neurovascular structures. Risks include infection or prosthetic damage leading to extrusion or rupture [14]. Some patients may additionally find the expansion process to be uncomfortable. For patients with immediate reconstructive needs for acquired defects following rectal cancer resection, this is not a commonly utilized form of reconstruction.
Biological Tissue Matrices
Biological tissue matrices are commercially available allograft or xenograft products. These can be derived from human (dermis), porcine (dermis and small intestine submucosa), or bovine (dermis and pericardium) sources [15]. In the setting of perineal reconstruction, biological tissue matrices are chiefly used to create pelvic diaphragms intended to limit visceral herniation and to reinforce abdominal donor site defects against bulges/herniations, especially in the context of prior radiotherapy or future ostomy placement. These biologic or acellular dermal matrices need to be placed next to well-vascularized soft tissue. Seromas in these regions can lead to infection. Closed suction drains need to be used in these types of reconstruction.
Characterization of Axial Pattern Flaps
Rectus Abdominis Muscle
The rectus abdominis muscle is harvested as the pedicled vertical rectus abdominis myocutaneous (VRAM) flap . It is supplied by the deep inferior epigastric system. This flap can be utilized as a muscle only, myocutaneous, or perforator flap with a large skin paddle, making it versatile for perineal reconstruction. Further increasing its utility is the ability to close all but the largest donor sites primarily, oftentimes with the help of a biological tissue matrix. Several studies have shown the VRAM is indispensable in eliminating large amounts of dead space, as would be seen in extensive APR defects [16, 17]. One group also found that despite VRAM complication rates being comparable to primary closure complication rates, defects repaired with VRAM flaps experienced drastically reduced rates of major wound dehiscence (9% vs 30%) and perineal abscess (9% vs 37%) [18]. This is secondary to reducing the dead space in the pelvis and recruiting vascularized soft tissue.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree