Rash Syndromes
Introduction
Rashes in children are often caused by a systemic infection and are frequently associated with fever. The same pathologic process that is producing the skin eruption can involve other parts of the body as well, such as the lungs, liver, and spleen. In fections of the skin are covered in Chapter 17.
Definitions
Exanthem is a term sometimes used to describe a rash associated with a systemic illness, especially with fever. The word exanthem comes from Latin and means a “breaking out” or “blossoming out” and implies a generalized illness accompanied by a rash. The word is less useful than “fever and rash” as a broad preliminary problem-oriented diagnosis.
Enanthem is sometimes used to describe an eruption on the oral mucosa. It usually represents the same pathologic process as the rash but occurring in the mouth.
Most viral exanthems are more prominent in areas exposed to the sun, especially if there is a sunburn.1
Classification
Rashes can be classified according to either the appearance of the rash itself (maculopapular, petechial, pustular) or the total illness pattern (rubella-like illness or scarlet fever-like illness). In this chapter, both forms of classification will be used. Within each category of rash, the principal specific causes will be described. It is a better and more specific problem-oriented diagnosis to say “scarlet fever-like illness” and reserve the broader diagnosis of “erythematous rash” for cases with few, if any, features of scarlet fever present.
Erythematous Rashes
Erythematous rashes (Box 11-1) usually are generalized and extensive but may involve only part of the body. They may resemble sunburn, with redness that blanches when pressed with the fingers but involving areas not exposed to the sun. They may be slightly papular or perfectly flat (macular).
Possible Etiologies
Scarlet Fever
The scarlet fever rash is produced by an erythrogenic toxin of Group A streptococci. Multiple toxins can produce the syndrome. One study demonstrated that strains of Group A streptococcus (GAS) that produced two or three different toxins caused a more intense rash. Those that produced erythrogenic toxin B caused illness with a higher fever.2 Scarlet fever can occur more than once in a single individual. Other than the rash, scarlet fever is no different from streptococcal pharyngitis. Therefore, the child typically will have clinical manifestations of streptococcal pharyngitis, as discussed in Chapter 2. Typically, these include headache, abdominal complaints, and a sore throat. Actual pharyngeal findings may be minimal. Suppurative and nonsuppurative complications may also occur. A study from Japan suggested that scarlet fever was associated more frequently with GAS serotype T4, which, in their study, was more difficult to eradicate from the pharynx than the other T types.3
Scarlet fever (scarlatina) is characterized by very small, often confluent, fine red papules and typically occurs on the trunk and extremities. In dark-skinned individuals, it is sometimes easier to diagnose by palpation than by vision. Patients often complain that the rash itches. There is increased redness in the folds of the skin, especially in the inguinal or antecubital creases, producing lines of redness called Pastia’s lines (Fig. 11-1). The face is typically flushed, and there is circumoral pallor. A “strawberry tongue” (rough and red), sometimes with a white coating, may be present. The skin typically feels slightly rough, like fine sandpaper. The rash of scarlet fever also involves areas of the body
usually covered by clothing, thus distinguishing it from ordinary sunburn. After about 7–10 days, the superficial layers of the skin may peel (desquamation), especially on the hands and feet. Occasionally, a localized area of streptococcal cellulitis is overlooked because of the generalized scarlet fever rash. Jaundice secondary to hepatocellular damage can complicate scarlet fever and may cause some diagnostic confusion if the physician is unaware of this possibility.4 Rare reports of autoimmune diseases such as guttate psoriasis occurring after scarlet fever suggest the possibility that, in some patients, superantigenic toxin stimulation of the immune system may lead to immune dysregulation.5
usually covered by clothing, thus distinguishing it from ordinary sunburn. After about 7–10 days, the superficial layers of the skin may peel (desquamation), especially on the hands and feet. Occasionally, a localized area of streptococcal cellulitis is overlooked because of the generalized scarlet fever rash. Jaundice secondary to hepatocellular damage can complicate scarlet fever and may cause some diagnostic confusion if the physician is unaware of this possibility.4 Rare reports of autoimmune diseases such as guttate psoriasis occurring after scarlet fever suggest the possibility that, in some patients, superantigenic toxin stimulation of the immune system may lead to immune dysregulation.5
BOX 11-1 Possible Causes of Erythematous (Scarlet Fever-like) Rashes
| Group A streptococcal infection Arcanobacterium haemolyticum pharyngitis Staphylococcal scarlet fever Staphylococcal scalded skin syndrome Drug of plant allergic reactions Erythema infectionsum involving cheeks Kawasaki disease Toxic shock syndrome Atropine, Vancomycin, and other drugs Atypical measles |
When there is no clinical evidence of a streptococcal pharyngitis, cellulitis, or other infection, other possible causes of scarlet fever-like (scarlatiniform) rashes should be considered.
Staphylococcal Scarlet Fever
Some strains of Staphylococcus aureus can produce a scarlet fever-like rash that has been called staphylococcal scarlet fever.6 Enterotoxins G and I seem to be involved in the pathogenesis of this syndrome.7 Unlike streptococcal scarlet fever, there is usually no circumoral pallor or strawberry tongue, and the erythematous skin is often painful or tender. As in streptococcal scarlet fever, desquamation of the superficial epidermis may occur after about a week. If the superficial skin separates and sloughs after only a few days, the patient should be classified as having scalded skin syndrome. This syndrome includes a group of illnesses, of which staphylococcal scarlet fever is a mild form, and is described further in Chapter 17.
Toxic Shock Syndrome (TSS)
This condition may be caused by Group A streptococcus or S. aureus. In toxic shock syndrome (TSS), the skin is flushed, and a scarlet fever-like rash may be noted. Fever, mild diarrhea, dizziness, and hypotension are common. Headache, sore throat, and severe myalgia are often present. Sometimes, confusion ensues. Misleading clinical features that may be present include a red swollen tongue; a generalized scarlet fever-like rash; nonpurulent conjunctivitis, sometimes with a subconjunctival hemorrhage; and mild subcutaneous edema. These clinical features may resemble Kawasaki disease, described later in this chapter. Herpes stomatitis or genital herpes may occur. All mucous membranes are typically very red. The focus of staphylococcal infection may be quite subtle, such as a mildly inflamed cervical lymph node. Tampon use is still a risk factor for staphylococcal TSS. Although cases associated with tampon use are less common today, they still comprise about half of all cases.
The white blood cell count (WBC) is typically greater than 15,000/mcL3 with many immature neutrophils. The hemoglobin may fall several grams, suggesting hemolysis. The sedimentation rate is very high. Urinalysis reveals proteinuria, pyuria, and mild hematuria, but there are few or no bacteria in the sediment. Other findings are described in more detail in Chapter 10. Diagnostic criteria are listed in Boxes 10-3 and 10-4.
Penicillin can be given without waiting for throat culture results in a patient with pharyngitis and a scarlet fever-like rash. If a wound infection is present and the patient appears seriously ill or if TSS
is suspected, intravenous nafcillin (or vancomylin) and clindamycin should be used (see Chapter 10).
is suspected, intravenous nafcillin (or vancomylin) and clindamycin should be used (see Chapter 10).
Arcanobacterial Scarlet Fever
Arcanobacterium haemolyticum has been recognized as a cause of pharyngitis and a scarlet fever rash, particularly in teenagers and young adults.8,9 An urticarial or erythema multiforme-like rash has been observed in some patients. Detection requires incubating sheep blood agar for at least 72 hours and observing for small alpha-hemolytic colonies; alternatively, human or rabbit blood agar can be used for more rapid detection. A. haemolyticum is generally susceptible to penicillin, the cephalosporins, and the macrolides. Whether antibiotic therapy speeds recovery from this self-limited illness is unknown. Rheumatic fever has not been reported in association with A. haemolyticum infection.9
The terms streptococcal, staphylococcal, and arcanobacterial should be used as adjectives to further define scarlet fever when possible.
Erythema infectiosum (“Fifth Disease”)
Usually a mild disease, erythema infectiosum is caused by human parvovirus B19. The rash has been aptly described to occur in three phases. Early in the illness, there is an erythematous rash on the cheeks (“slapped cheeks”), resembling that seen in scarlet fever. Later, there is a macular or maculopapular, variably pruritic red rash that favors the extensor surfaces of the limbs; it starts as solid red areas, but as it progresses the middle parts clear in a seemingly random fashion, leading to the classic lacy, reticular appearance (Fig. 11-2), which typically lasts 2–5 days. The third phase of the rash is recurrence. Patients and their families should be informed that the rash may reappear after it is seemingly gone. Recurrences are sometimes associated with environmental triggers such as hot water or sunlight.
Typically, the patient with erythema infectiosum is not sick and seeks medical attention only because of the rash. Arthralgia may occur but is much more common in adults. Outbreaks are common. Complications such as encephalitis are rare.10 Other clinical conditions that have been reported to occur in association with erythema infectiosum include acute cerebellar ataxia,11 Guillain-Barré syndrome,12 hepatitis13 or fulminant liver failure,14 and chronic fatigue syndrome.15 Proof of causality is lacking for most of these conditions, although parvovirus B19 DNA was found by polymerase chain reaction (PCR) in the serum of some patients with idiopathic hepatitis and was found in liver biopsy specimens of some patients with fulminant hepatic failure.
 FIGURE 11-2 Erythema infectiosum (fifth disease) showing typical gyrate rash in A and other appearances of the rash in B and C. (Balfour HH Jr. Clin Pediatr. (Phila) 1969;8:721–7). |
The historical background of fifth disease is of some interest. About 1900, there was a controversy over the number of rash diseases that might occur in epidemics. At a boys’ school in Rugby, England, Dukes observed what he thought were two different rash diseases and wrote an article entitled “Confusion
of Two Diseases under the Name of Rubella (Rose-Rash).” Another physician questioned Dukes’s observations in an article entitled, “Scarlet Fever, Measles, and German Measles—Is there a Fourth Disease?”16 Dukes claimed there was a fourth disease on the basis of the absence of repeated attacks at the boys’ school. In the modern era, medical history scholars have concluded that this “fourth disease” described by Dr. Dukes probably never existed. Rather, these were likely to have been cases of misdiagnosed rubella and scarlet fever.17 Nevertheless, Dukes’s article led to the naming of various exanthems as “fifth disease,” “sixth disease,” and “seventh disease.” Of these numbered diseases, only the term fifth disease is still sometimes used as a synonym for erythema infectiosum. Sixth disease probably was roseola infantum, described later in this chapter.
of Two Diseases under the Name of Rubella (Rose-Rash).” Another physician questioned Dukes’s observations in an article entitled, “Scarlet Fever, Measles, and German Measles—Is there a Fourth Disease?”16 Dukes claimed there was a fourth disease on the basis of the absence of repeated attacks at the boys’ school. In the modern era, medical history scholars have concluded that this “fourth disease” described by Dr. Dukes probably never existed. Rather, these were likely to have been cases of misdiagnosed rubella and scarlet fever.17 Nevertheless, Dukes’s article led to the naming of various exanthems as “fifth disease,” “sixth disease,” and “seventh disease.” Of these numbered diseases, only the term fifth disease is still sometimes used as a synonym for erythema infectiosum. Sixth disease probably was roseola infantum, described later in this chapter.
The etiology of the disease was in doubt for some time, but parvovirus B19 has been clearly established as the etiologic agent of erythema infectiosum. Serologic studies of outbreaks were the first to support causality.18 Subsequently, PCR studies demonstrated viral DNA in plasma samples of patients with erythema infectiosum.
Erythema infectiosum had an incubation period of 4–12 days in a Canadian outbreak18 and of 13–18 days in a 1985 New York state cluster.19 In the Canadian outbreak, respiratory and systemic symptoms were more prominent, the rash occurred on the palms and soles, and facial erythema and a lacy rash were less prominent than in previously published cases.18
The rash appears to be preceded by a viremic phase, with the virus present later in respiratory secretions. The contagious period is not clearly established, but patients are generally not contagious by the time the rash is obvious. In a small but thorough study in Canada, the attack rate in adults was only 14%.18 The epidemiology is somewhat confusing in that there appear to be peaks of disease that last 3 years and occur every 6 years. The frequency of infection in susceptible adult household contacts is about 50%.20
Erythema infectiosum has always been regarded as a benign illness in normal children. The virus attachment protein is erythrocyte antigen P (globoside). This protein is expressed in largest amounts on erythrocyte precursor cells. Hence, infection with parvovirus B19 shuts down the production of red cells in the bone marrow and reduces the reticulocyte count to near zero. This transient shutdown of red cell production is not a problem for patients with normal hemoglobin. However, patients with sickle cell anemia or other hemoglobi nopathies, who are dependent on maintaining a high reticulocyte count, can experience aplastic crises when infected with parvovirus. These crises can be severe and even life threatening. Patients with aplastic crises should be considered to be contagious. Polyarthritis can occur in adults during outbreaks of the rash in children, and laboratory results also support a role for parvovirus in arthritis.21 Chronic bone marrow failure with persistence of parvovirus B19 in the marrow has been reported in patients with organ or bone marrow transplants,21a malignancies,21 combined immunodeficiency,22 and humoral immunodeficiencies.23 This condition may respond to intravenous immunoglobulin (IVIG) therapy.21a Transient acute pancytopenia may occur in normal individuals.
Parvovirus infection during pregnancy is one of the most common causes of nonimmune hydrops fetalis (see Chapter 19). Hydrops is more likely to occur when maternal infection occurs during the second trimester.24 Seropositivity rates in women of childbearing age range from 35%25 to 65%26; annual seroconversion rates are about 1.5%, but may be as high as 13% in epidemics.26 The risk of seroconverting during pregnancy is higher with increasing numbers of children in the home; nursery school teachers have about a three-fold increased risk of acquiring infection during pregnancy.26 When primary maternal infection does occur, the fetus is often infected. However, most cases are asymptomatic. One prospective study of 43 women who were infected during pregnancy documented that 51% of the fetuses became infected; however, all the babies were carried to term and were born healthy.27 In another prospective study of 1,610 women, 60 became infected during pregnancy (3.7%); only one fetus was aborted secondary to parvovirus infection, yielding a risk of fetal loss of 1.7% in known infection.25 The following calculations are helpful in counseling pregnant women who are exposed to parvovirus B19: approximately 50% of women of childbearing age are susceptible, approximately 30% of susceptible hosts become infected, approximately 25% of exposed fetuses become infected, and approximately 10% of infected fetuses die. Thus, the risk of fetal death in a woman exposed to parvovirus B19 is about 0.5 × 0.3 × 0.25 × 0.1 = 0.4%.
Other Causes
Drug reactions occasionally produce an erythematous rash; for example, the pharmacologic effect of atropine, rapid vancomycin infusion, or a reaction to ampicillin. Chickenpox may have a transient erythematous appearance before the vesicular eruption. Many allergic reactions result in erythematous rashes.
Erythromelalgia is a rare episodic disease of unknown cause manifested by attacks of redness and pain in the hands and feet that is relieved by immersion in ice water or by aspirin.28 Contact dermatitis, such as from plants, can produce erythematous rashes often associated with itching and papules or vesicles.29 Kawasaki disease (KD) should be included in the differential diagnosis of scarlet fever-like rashes but is given its own section because of its importance.
Kawasaki Disease
This disease was named in honor of Tokyo physician Tomisaku Kawasaki, who first reported it in the United States’ literature in 1974.30 Most of the children came to his attention during first 7 years of the 1960s. He called the syndrome the mucocutaneous lymph node syndrome because of the erythema of the eyes and mouth and either generalized or focal lymph node enlargement. The first cases in the United States were observed in 1971 in Hawaii, and it was first reported in the continental United States in 1976. Now that the disease is well described and known, it is not an uncommon diagnosis.
Epidemiology
The disease occurs almost exclusively in children, usually those less than 4 years of age, and is more severe in Asian children, who may have a fatality rate of 1–2% in severe cases. Sporadic case reports of a similar disease in adults have been appearing. There are over 6,000 cases per year in Japan, for an average annual incidence of 105 cases per 100,000 children under 5 years of age.31 In San Diego, the disease was found to have a higher incidence in the coldest and rainiest months. Incidence was also seen to be higher in those of Asian or Pacific Island descent.32 The disease in Japanese children is significantly more frequent in those with HLA antigen BW22. A recent case-control study suggested that the incidence was higher in the children of health care workers.33 This finding is interesting but requires confirmation.
Etiology
Despite a resolute effort, the etiology of the disease remains elusive. Because the disease resembles toxin-mediated diseases such as scarlet fever and toxic shock syndrome, some researchers have assumed a bacterial cause; some have demonstrated staphylococci that overproduce enterotoxin B34 or protein A35 and have suggested, therefore, that staphylococci with mutations in the accessory gene regulation locus are responsible for the disease.36 Unfortunately, it has not been easy to find staphylococcal infection in all cases of KD. Others have claimed streptococci as the cause, but a series of children with a history of KD had no antibodies to streptococcal superantigens.37 A link to Chlamydia pneumoniae infection was sought but not established. Features of the disease resemble superantigen-mediated illness, but this has not been clearly established; even if it were, delineating the exact source and nature of the superantigen would require much more work.
At present, it is not entirely clear that KD is actually an infectious disease. Clustering of cases, however, suggests that it probably is. It may be that the disease is a final common pathway of multiple infectious insults, rather than being attributable to any one etiologic agent.
Pathophysiology
The clinical findings of KD are the result of the release of multiple inflammatory mediators. In the acute phase of the illness, vascular endothelial growth factor (VGEF),38 monocyte chemotactic protein 1 (MCP-1),39 and soluble CD4 levels40 are increased in patients with KD. Some of these mediators have been linked to specific manifestations of the disease. For example, VGEF levels are highest in children who develop coronary artery abnormalities38 and MCP-1 has been found in cardiac tissues of children who died of KD.41 It is possible that there is a genetic predisposition to the disease. Peripheral blood mononuclear cells from patients who long ago recovered from KD overexpress TNF-alpha in vitro compared with cells from patients
without disease history.42 Anticardiac myosin antibodies have also been demonstrated in the sera of patients suffering from acute KD,43 which may help explain the propensity of the disease to damage vessels and specifically to damage the arteries that feed the heart.
without disease history.42 Anticardiac myosin antibodies have also been demonstrated in the sera of patients suffering from acute KD,43 which may help explain the propensity of the disease to damage vessels and specifically to damage the arteries that feed the heart.
Clinical Presentation
Kawasaki disease may resemble scarlet fever because of the fever, the generalized erythematous rash, the red oral mucosa and tongue, a marked leukocytosis with a shift to the left, and later, the desquamation of the fingertips and toes. Nonsuppurative cervical adenitis may be prominent, but is the most frequently absent of the classic findings. The fever lasts 5–14 days and does not respond to penicillin or other antibiotics.
The Centers for Disease Control and Prevention (CDC) has defined criteria for the case definition Box 11-2.44 It is important to remember that this case definition was created for epidemiologic purposes, not clinical ones. Epidemiologic case definitions are meant to be specific (strict), so that patients with other diseases do not end up being incorrectly classified as having the disease of interest. The trade-off is that such definitions may lack sensitivity. Such is the case with KD. If only patients meeting the CDC criteria are diagnosed with KD, cases will be missed. Often, only three or four of the characteristics of KD are found, causing a great diagnostic difficulty. Swelling of hands and feet is a very helpful diagnostic point and may sometimes be discovered only by the history. The rash is usually nonspecific, generalized, and maculopapular, but may take almost any form, including one that resembles erythema multiforme. The rash tends to be most intense in the diaper area. Involvement of the conjunctivae is one of the most constant features of the disease. The sclerae tend to be injected with many bright red vessels. The limbus is spared. There is almost never a purulent discharge. Clinical experience teaches that children (especially toddlers) with KD are usually extremely irritable. The fever is normally quite high and responds poorly, if at all, to antipyretic administration.
BOX 11-2 Case Definition of Kawasaki Disease
| Fever for 5 or more days Plus four of the following five clinical findings:
|
| Plus exclusion of other causes |
A mild myocarditis is common at the time of presentation, and careful auscultation will reveal gallop rhythms in some patients. Despite this, fluid tolerance is normally good, and even patients with gallops tolerate the fluid load associated with IVIG administration. Hydrops of the gallbladder is less frequently seen (about 10% of cases), but its presence suggests KD in the appropriate clinical setting.45,46 Abdominal ultrasound, therefore, is sometimes helpful is paring down the differential diagnosis. Similarly, anterior uveitis (iritis) can be detected on slit lamp examination in > 50% of patients, and its presence is supportive of KD in the child with features consistent with the diagnosis.47 If the diagnosis of KD is clinically obvious, lumbar puncture is not necessary. A retrospective review of patients eventually shown to have KD who underwent spinal tap at presentation in the ER showed that almost 40% of them had a mild cerebrospinal fluid (CSF) pleocytosis (mean of 23 WBC, 6% neutrophils). About 17% had an elevated protein, but hypoglycorrhachia was not associated with the disease.48 In several different cases, children with KD presented with a peritonsillar phlegmon or abscess.49 Children from other countries who received BCG in the newborn period occasionally develop granulomatous inflammation at the site of the BCG during acute KD.50
Differential Diagnosis
KD may be mistaken for Stevens-Johnson syndrome (see following) because of the rash; conjunctivitis; red oral mucosa with red, dry, fissured lips; and failure to respond to antibiotics. The rash also can resemble erythema multiforme, with central clearing and iris lesions.51 However, the conjunctivitis in KD is nonpurulent.
Other diseases resembling KD include Rocky Mountain spotted fever (more common during the summer months)52 and leptospirosis.53 Toxic
shock syndrome (Chapter 10) caused by either S. aureus or S. pyogenes may also resemble KD. Systemic onset juvenile rheumatoid arthritis (Chapter 10) may mimic KD, as may adenoviral infection.
shock syndrome (Chapter 10) caused by either S. aureus or S. pyogenes may also resemble KD. Systemic onset juvenile rheumatoid arthritis (Chapter 10) may mimic KD, as may adenoviral infection.
Laboratory Studies
Typically, there is a marked leukocytosis with a shift to the left, slight anemia, thrombocytosis (a useful later finding), elevated C-reactive protein (CRP), increased alpha2-globulin, and a markedly increased erythrocyte sedimentation rate (ESR). The serum IgM and IgE are typically elevated.54 The serum IgE often is persistently elevated if arthritis or cardiac complications occur. Mild jaundice or slightly increased serum amino-transferases occur.
Additional Findings and Complications
Often, the patient will have or develop prominent findings other than those in the case definition. These include diarrhea, focal ileus, myositis, malabsorption, arthralgia or arthritis, uveitis, hemolytic-uremic syndrome, hyponatremia, proteinuria, sterile pyuria, and others listed in Box 11-3.55,56,57,58,59
BOX 11-3 Kawasaki Disease: Additional Findings and Complications
| Cardiac Acute myocarditis Acute mitral insufficiency Coronary artery aneurysm or thrombosis (later) Pericardial effusion |
| Other Nonpurulent meningitis Hydrops of gallbladder Obstructive jaundice Diarrhea, abdominal pain, ileus Pancreatitis (later) Arthralgia, arthritis Sterile pyuria, urethritis Uveitis, frequent and mild Parotitis Coombs-positive hemolytic anemia Hemolytic-uremic syndrome Retropharyngeal lymphoid mass Myositis, weakness due to hypokalemia Aseptic necrosis of bone (later) Hyponatremia Psoriatic skin lesions Distal limb ischemia Facial nerve palsy |
Aneurysm or thrombosis of the coronary arteries (resembling, but different from, infantile polyarteritis nodosa) is the most frequent of the severe complications.60 Myocardial infarction and sudden death can occur.
Echocardiography may be useful to detect coronary artery aneurysms. However, the timing of a cardiac evaluation can be a source of uncertainty to the physician. In the great majority of patients who will develop coronary artery aneurysms, early echocardiographic signs are present by 10–14 days after illness onset.61 Consequently, the American Heart Association (AHA) recommends an initial echocardiogram at 10–14 days. The initial appearance of aneurysms more than 6 weeks after onset is very uncommon.62 Thus, the AHA guidelines call for a second echocardiogram 6–8 weeks after illness onset.61 A third echocardiogram is sometimes performed 6–12 months after illness onset if there is any concern about the previous studies.
Facial nerve palsy is an extremely rare complication of KD, but is of importance because of a possible association with coronary artery aneurysms; of 25 cases reported in the literature, more than half also had coronary artery aneurysms.63
Complications of treatment occasionally develop. One patient who developed aseptic meningitis in response to IVIG therapy has been reported.64 We have also cared for a patient who developed an infusion reaction that resulted in a fever spike to 105°F and a febrile seizure. The theoretical possibility of the development of Reye’s syndrome exists because of long-term aspirin therapy during the months when both varicella and influenza infection are common. However, this has never been reported. When feasible, influenza vaccination should be administered. Varicella vaccination can also be given but may not be effective for several months after receipt of IVIG. The recommended time interval between IVIG and varicelia or measles vaccination is 11 months in the United States and 6–7 months in Japan. A recent study suggests that even for patients who require repeat dosing (i.e., a total of 4g/kg), 9 months was a sufficient interval.64a
Treatment
High-dose IVIG is the most effective treatment, both in ameliorating the signs and symptoms and in preventing the cardiac consequences of the disease. The incidence of coronary artery abnormalities following KD has been shown to be inversely related
to the dose of IVIG received.65 For this reason, dosages less than 2 gm/kg are not recommended. The physician should attempt to make the diagnosis and start appropriate therapy as expeditiously as possible. Prospective studies of IVIG therapy for KD have included only those patients diagnosed within 10 days of the onset of fever. However, retrospective data suggest that for patients who are diagnosed later, IVIG therapy is still beneficial after the 10th day of illness.66 Most experts recommend administering IVIG to such patients if there are signs of ongoing inflammation.67
to the dose of IVIG received.65 For this reason, dosages less than 2 gm/kg are not recommended. The physician should attempt to make the diagnosis and start appropriate therapy as expeditiously as possible. Prospective studies of IVIG therapy for KD have included only those patients diagnosed within 10 days of the onset of fever. However, retrospective data suggest that for patients who are diagnosed later, IVIG therapy is still beneficial after the 10th day of illness.66 Most experts recommend administering IVIG to such patients if there are signs of ongoing inflammation.67
High-dose aspirin (80–100 mg/kg/day divided in four doses) has been advocated for the acute part of the illness, which is variously interpreted as a duration of 2 weeks, until the patient is afebrile, or until the CRP normalizes. In the study of outcomes by IVIG dosage cited earlier, coronary artery abnormalities were shown to be unrelated to the dosage of aspirin.65 Japanese physicians use 30–50 mg/kg/day as their high-dose standard. In the past, serum levels of aspirin were monitored, and a level of 20 mg/dL or greater was targeted; reaching those levels often required as much as 100–180 mg/kg/day. There is no evidence that serum aspirin levels predict outcome. Consequently, frequent aspirin level measurements and dosage adjustments seem unwarranted.
After the acute period of the disease, low-dose aspirin is used to reduce the risk of coronary artery thrombosis. Most American physicians use 3–5 mg/kg/day in a single dose. Low-dose aspirin is continued until the ESR normalizes, which usually takes 1–3 months. Some believe that even if the ESR normalizes sooner, low-dose aspirin therapy should be continued no less than 8 weeks.68 Alternatively, the most practical approach is probably to discontinue the aspirin if the echocardiogram done 6–8 weeks after the illness is negative.
The use of corticosteroids has never been studied in a controlled fashion, but there is some suggestion that they are associated with an increased risk of coronary artery aneurysm. On the other hand, they are sometimes used in the patient who fails to respond to two doses of IVIG.69
Prognosis
The most important predictor of outcome is whether the patient receives IVIG or not. The rate of coronary artery aneurysms at 7 weeks in patients who do not receive IVIG is 15%.71a In contrast, if IVIG is administered within 10 days of illness onset, the rate is 2.4%.71b
If coronary artery aneurysms do not occur, the prognosis is excellent. Most patients respond quite dramatically to the infusion of IVIG. In some cases, parents will describe how they watched the conjunctival injection disappear right before their eyes as the infusion progressed. However, there is a significant minority of patients who do not respond promptly to IVIG therapy. Many of them respond to a second administration. Investigators have sought clinical or laboratory parameters that would be predictive of a poor response to the first dose of IVIG. Results have been mixed. Some have found that a CRP greater than 10 mg/dL, an LDH greater than 590, and a hemoglobin of less than 10 g/dL were predictive both of poor response to therapy and of high risk for the development of coronary artery aneurysms.72 Others have not been able to identify any features of either the disease pattern or laboratory values that reliably predict response to therapy.
The following have been identified as placing the patient at high-risk for coronary artery aneurysms: duration of fever greater than 9 days prior to IVIG therapy,73 age less than 1 year or greater than 8 years,74 highly elevated CRP level, presence of pericardial effusions or ventricular dysfunction at presentation, and a recurrent case.75 A poor response to the first dose of IVIG, including failure to become afebrile within 48 hours or recrudescence of fever after becoming afebrile,76 elevated WBC count, absolute neutrophil count, or CRP level after IVIG infusion have also been associated with the development of coronary artery aneurysms.
About half of all coronary artery abnormalities that are discovered within the first few days of treatment regress and never reform.77
Incomplete Kawasaki Disease
The disease is harder to recognize in patients less than 6 months or greater than 8 years of age, in the former because it tends to occur in an incomplete form and in the latter largely because it is unexpected in that age group. In either case, the prognosis is worse than in children of the ages in between, who have a more readily recognizable form of the disease. Children who have incomplete or atypical KD, therefore, are at higher risk of developing coronary artery disease.78 Echocardiography
has therefore been recommended for children who have a long unexplained febrile illness with subsequent desquamation.79,80 Because delayed in diagnosis and treatment may be at least partially responsible for the increased incidence of coronary artery aneurysms in infants with incomplete KD, the diagnosis should be considered in patients with clinical and/or laboratory findings noted in Box 11-4.
has therefore been recommended for children who have a long unexplained febrile illness with subsequent desquamation.79,80 Because delayed in diagnosis and treatment may be at least partially responsible for the increased incidence of coronary artery aneurysms in infants with incomplete KD, the diagnosis should be considered in patients with clinical and/or laboratory findings noted in Box 11-4.
Measles-like Illnesses
Measles-like illness has been defined for use in epidemic control by the CDC as a very broad syndrome:
Fever of 101°F or higher
Rash lasting 3 or more days
One or more of the following: cough, conjunctivitis, or coryza
The preceding definition is useful in measles vaccination campaigns in areas of the world where measles is common. In the United States, the vast majority of children with symptoms meeting this definition will not have measles. The spectrum of measles virus infection is described in this section using the terms classic, vaccine-modified, and immunoglobulin-modified. Other maculopapular rashes that may resemble measles are described in the next section. Rubeola is an older name for measles that one hopes will become obsolete, because it is easily confused with “rubella” (which is unfortunately sometimes referred to as German measles).
Box 11-4 Clinical and Laboratory Findings Suggestive of Possible Incomplete Kawasaki Disease
| Clinical findings Daily high spiking fevers for 5 days or longer without evidence of bacterial infection, with either:
|
Laboratory findings
|
| From Rowley AH. Incomplete (atypical) Kawasaki disease. Pediatr Infect Dis J 2002;21:563–5 |
Classic Measles
Epidemiology
Measles was once a very common childhood disease in the United States; an average of 500,000 cases a year were reported in this country before widespread vaccination took place. Serologic surveys in military recruits in the late 1960s found that almost all were measles antibody positive. Disease occurred in epidemics every 2–5 years. Epidemics lasted from 2–4 months. In temperate climates, measles was a disease of the late winter and spring. In the prevaccine era, elementary school children were most often infected. Measles is a highly contagious disease. The virus spreads very rapidly through a population, infecting all susceptible individuals. In the developing world, measles remains a leading cause of preventable illness; it causes 800,000 deaths each year in children < 5 years old.81
Killed virus vaccine was introduced first, and then attenuated live virus vaccine was introduced. The vaccine is highly effective, inducing durable protection in approximately 95% of vaccinees with a single dose. Widespread use of measles vaccine caused the incidence of epidemic measles to drop rapidly. By 1968 the number of cases of measles reported to the CDC had decreased by more than 90% compared with the number reported in 1964. However, over a 3-year period from 1988–1991 the incidence of measles jumped up again; there were 27,786 reported cases in 1990. This resurgence of measles cases prompted the recommendation for two doses of live, attenuated measles virus vaccine. The principal purpose of the second dose of vaccine is to produce immunity in subjects who failed to make a response to the first dose of vaccine, rather than to “boost” immunity in those who responded; however, there is some evidence that protection against disease is better in those who have received two doses of vaccine.82 Additionally, avidity studies of antibodies in vaccinees show that in outbreak situations, some symptomatic measles infections occur in patients who were vaccinated
years prior to the epidemic.83 This effect was most pronounced among those who received vaccine prior to their first birthday. The two-dose measles vaccine schedule appears to be working: the number of reported measles cases in the United States during both 2001–2003 was 216, which is an all-time low and represents an incidence of less than 0.3 cases per one million population.84
years prior to the epidemic.83 This effect was most pronounced among those who received vaccine prior to their first birthday. The two-dose measles vaccine schedule appears to be working: the number of reported measles cases in the United States during both 2001–2003 was 216, which is an all-time low and represents an incidence of less than 0.3 cases per one million population.84
Clinical Presentation
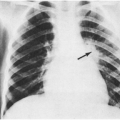
Classic measles is a moderate to severe illness with fever of 39.5–40.6°C (103–105°F) and severe cough and conjunctivitis for several days before the appearance of the rash. The early problem-oriented diagnoses (before the rash appears) are likely to be febrile bronchitis and nonpurulent conjunctivitis. The incubation period between the exposure and the first symptoms is 8–12 days, averaging about 10 days. The rash typically begins on the face and neck and spreads downward to involve the entire body. The rash is extensive and confluent and lasts about 7 days. It tends to be more confluent in the areas first affected and less so in the extremities. A small amount of desquamation may occur as the rash clears. Fever persists for several days after the onset of the rash and then breaks abruptly. An enanthem (Koplik spots) often precedes the rash by a day and consists of white papules the size of a small pinhead on an erythematous base on the buccal mucosa. Koplik’s spots are diagnostic of measles. A generalized enanthem is often found during the peak of the rash. Cough, rhinitis, and conjunctivitis are prominent (Fig. 11-3). The conjunctivitis usually produces a copious, watery discharge, and older patients sometimes describe photophobia. Lymphadenopathy may be prominent, especially in head and neck. Mortality is higher in developing countries with crowded conditions or with more than one case in a household.
Modified Measles
This is a technical term defined as measles virus infection made milder by previous antibodies. The term was commonly used when immunoglobulin (IG) was given to exposed susceptible siblings before measles vaccine was available, from about 1950 to 1967. Mild to moderate measles-like illness can have several causes, but in an endemic area measles virus infection modified by antibodies is the commonest. These antibodies may be transplacental maternal antibodies, which are present in the first year of life, or be derived from recently administered serum immune globulin. A modifying dose of IG (0.05 ml/kg) prolongs the incubation period and makes the illness milder. The traditional view was that transplacental antibodies prevented disease in the infant in the first 6 months of life and allowed a modified measles illness between 6 and 12 months of life. The term modified measles has also been used to describe mild measles confirmed by serologic testing in individuals who have received one or another form of measles vaccine (Table 11-1).85
Revised Terminology
Because of the difficulty of using traditional terminology to describe the live-vaccine alteration of measles virus infections, a very useful classification has been proposed.86 In this classification, “classic measles” is infection with wild measles virus. Vaccine-modified measles is then subdivided into killed or live vaccine modification, which seem to have different patterns, the former with a more severe course and an atypical rash and the latter with a milder course much like immune globulin-modified measles. The term modified measles as used earlier is then changed to “immunoglobulin-modified measles” (see Table 11-1).
Killed Vaccine Modified Measles (“Atypical Measles”)
Contracting measles after receipt of the killed virus vaccine caused a variant form of measles that was difficult to diagnose because it often lacked classic features such as conjunctivitis, coryza, and Koplik’s spots. The rash was also altered, sometimes being vesicular or urticarial. Killed measles vaccine has not been used in over three decades, so atypical measles is no longer seen.
Live-Vaccine Modified Measles
After a person who has been immunized with live attenuated measles vaccine is exposed to the wild virus, there are three possible results. In at least 90% of exposures, the individual will be completely protected and get no illness at all. Rarely, the individual will get classic measles, indicative of a “vaccine failure,” sometimes attributable to improper storage of vaccine, use in children younger than 12 months, or use with immune globulin, as was sometimes done in the 1960s. Finally, a live-vaccine-modified measles can occur, with a shorter,
milder measles-like illness. This pattern resembles the killed vaccine-modified pattern (“atypical measles”) but does not seem to have any focal pneumonia or atypical rash attributable to an antigen–antibody reaction. Live-vaccine-modified measles more nearly resembles immunoglobulin-modified measles; that is, with all the features of classic measles made shorter and milder.
milder measles-like illness. This pattern resembles the killed vaccine-modified pattern (“atypical measles”) but does not seem to have any focal pneumonia or atypical rash attributable to an antigen–antibody reaction. Live-vaccine-modified measles more nearly resembles immunoglobulin-modified measles; that is, with all the features of classic measles made shorter and milder.
 FIGURE 11-3 Faces of two children with classic measles. Note eye swelling, nasal discharge and obstruction, mouth breathing, and rash (Photos from Dr. Robert Lawson). |
TABLE 11-1. FORMS OF MEASLES | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
Diagnosis of Classic Measles
The diagnosis is usually a clinical one. In the setting of an outbreak, diagnosis is relatively easy. Because many currently practicing physicians have never seen a case of measles, this disease, once fairly reliably diagnosed by parents, may escape diagnosis in some cases. Serologic studies are available when the diagnosis is suspected, but in doubt; hemagglutinin-inhibition methods are the most reliable. IgM is usually detectable for about a month after the rash begins. Acute and convalescent titers that show a fourfold or greater rise in the setting of clinical measles may be considered diagnostic. Nasopharyngeal swab specimens or urine may be sent off for viral culture. The physician should contact their state laboratory before collecting specimens.
Treatment of Classic Measles
Specific antiviral therapy directed against measles virus has not been shown to be efficacious in the treatment of measles. Ribavirin is active against measles virus in vitro and is used in the treatment of severe measles virus disease in immune-compromised hosts. Anecdotal, uncontrolled reports of cure in patients with severe measles virus pneumonia treated with ribavirin87 or the combination of ribavirin and IVIG88 have been published. Experimental antiviral agents that show greater activity against measles virus in vitro89 and in the cotton rat model90 are not yet available, nor are they recommended.
Acute measles virus infection takes a more serious course in patients who have vitamin A deficiency, and vitamin A levels are depressed during disease even in patients of normal nutritional status.91 Severity of clinical illness correlates inversely with vitamin A levels. Supplementation of vitamin A during acute disease hastens recovery. A large, retrospective study from South Africa is revealing: The authors reviewed 1,061 cases of measles prior to their use of vitamin A and compared these cases with 651 seen after the institution of vitamin A therapy for measles. Length of hospital stay (10 vs. 13 days), requirement for intensive care (4.3% vs. 10.5%) and mortality (1.6% vs. 5%) were all significantly reduced in patients who received vitamin A.92 The current recommendation is for patients between the ages of 6 months and 2 years of age who are hospitalized with measles to receive one dose of vitamin A (100,000 units for children 6 months to 1 year of age, and 200,000 units for children older than 12 months). Children who have eye findings suggestive of a preexisting vitamin A deficiency should get another dose the next day and another one at 4 weeks.
Complications of Classic Measles
Common Respiratory Complications
Croup may complicate measles and is sometimes severe and occasionally fatal. Cervical lymphadenitis and bacterial pharyngitis are common. Otitis media may occur and is usually nonbacterial, being related to lymphoid hyperplasia obstructing the eustachian tube.
Interstitial Pneumonia
Radiographic interstitial pneumonia is common during measles virus infection. Sometimes, it is complicated by secondary bacterial pneumonia. In an outbreak in St. Louis in 1970, pneumonia necessitating hospitalization was about ten times as frequent as encephalitis and had a 10% mortality rate.
Acute Measles Encephalitis
This complication occurred in about 1 of 1,000 to 10,000 reported cases of measles during an outbreak when measles was common. It is associated with a mortality rate of about 10%. Some degree of neurologic damage, such as convulsive disorder or mental retardation, follows in about 50% of cases. Cell-mediated immunity is probably most important in protection from the development of encephalitis. In a mouse model, CD8 cytotoxicity correlates with protection.93 Laboratory studies of a patient who was immunosuppressed because of therapy for ankylosing spondylitis and who eventually died of measles encephalitis revealed intact humoral immunity but virtually no cell-mediated immunity.94 Patients with pure humoral immune deficiency syndromes such as Bruton’s agammaglobulinemia
almost always recover from measles uneventfully. This makes sense given that measles is an enveloped virus, and therefore infected cells can produce progeny virions without being lysed. Ultimately, clearance of these infections usually requires active lysis by the host’s immune system. Measles encephalitis typically begins sometime during the period when the rash is present. About half of the children with measles encephalitis in the 1970 St. Louis outbreak eventually required custodial care.
almost always recover from measles uneventfully. This makes sense given that measles is an enveloped virus, and therefore infected cells can produce progeny virions without being lysed. Ultimately, clearance of these infections usually requires active lysis by the host’s immune system. Measles encephalitis typically begins sometime during the period when the rash is present. About half of the children with measles encephalitis in the 1970 St. Louis outbreak eventually required custodial care.
Fatal Giant-Cell Pneumonia
In immunocompromised patients, such as those with leukemia, there may be a fatal progressive bilateral pneumonia. In such cases, giant cells are typically found at autopsy. The measles may occur without a rash. In one report of four immunocompromised patients who developed giant cell pneumonia during acute measles virus infection, autopsy revealed the concomitant occurrence of pancreatitis, sialoadenitis, and thyroiditis.95 Occasionally, this pneumonia occurs in otherwise-normal hosts.
Dissemination of Tuberculosis or Fungal Infections
During measles, the virus may impair cell-mediated immunity, and unrecognized tuberculosis or pulmonary infection with fungi may disseminate. This dissemination is typical in countries where unrecognized tuberculosis is common.
Acute measles virus infection and vaccination with measles-mumps-rubella (MMR) vaccine can temporarily suppress tuberculin reactivity. A TST can be given during the same visit that MMR is given. However, if tuberculin testing is indicated and cannot be performed at the same time as MMR immunization, tuberculin testing should be deferred for 4–6 weeks.96
Subacute Sclerosing Panencephalitis (SSPE)
This condition occurs about 2 to 10 years after the initial infection and represents a latent measles virus infection in the brain. The incidence of SSPE following wild-type measles infection is somewhere between 0.5 and 2.0 per 100,000 cases. Attempts to define risk factors for the development of SSPE have been difficult; most experts believe that earlier acquisition of measles virus infection leads to higher risk.97 Usually, the disease begins with subtle mental changes and poorer performance in school and progresses to an intractable convulsive disorder. Rare presentations include chorioretinitis98 and optic neuritis.99 The condition progresses inexorably to death within a year or two.
Whether SSPE can occur after vaccination with MMR vaccine is controversial. If it does, it is extremely rare.
Encephalopathy in Children with Malignancies
Children with leukemia or neuroblastoma can develop a chronic progressive encephalopathy, apparently caused by the measles virus, during the period of acute measles infection.
Other Maculopapular Rashes
Many infectious and noninfectious illnesses can produce a rash resembling measles. In such a case, it is called morbilliform or measles-like. A maculopapular rash has elevated red bumps (papules) and red flat areas (macules). The individual macules and papules may become confluent after a day or so.
Papular rashes are described in a later section of this chapter.
Causes and Manifestations
Severe Rubella
Rubella virus infection can produce a moderately severe measles-like illness in adolescents and young adults. Prodromal respiratory symptoms, cough, rhinitis, conjunctivitis, pharyngitis, palatal petechiae, and a rash lasting longer than 5 days have been observed in laboratory-confirmed rubella virus infections in this age group.100 Pain on motion of the eyes, chills, and fever have been observed in young adults with proven rubella virus infection.101
Unknown Agents
In communities where live measles vaccine has been used extensively, measles-like illnesses are sporadic and should be studied serologically to determine if measles virus is the cause. In one study, measles virus infection could not be demonstrated serologically in 22 of 32 illnesses clinically compatible with measles, and it was suggested that other unidentified agents, presumably viruses, produced the illnesses, including some with an enanthem thought to be Koplik spots.102
Rubella-like Illness
This condition is defined as a mild illness with minimal fever or respiratory symptoms. Generalized lymphadenopathy and a generalized rash that lasts about 3 days are essential to the clinical diagnosis. Laboratory studies are extremely important if exposure of a pregnant woman is involved, as described in Chapter 19.
Rubella virus is the most important cause of rubella-like illness. Classical rubella virus infection is usually a mild disease with little fever. The rash appears about 14–21 days after the exposure, is nonconfluent and maculopapular, and lasts about 3 days. Generalized lymphadenopathy, particularly behind the ears (postauricular) and at the back of the head (occipital), is usually present. Mild splenomegaly may also be present. Rubella virus can be cultured from skin biopsies taken from the rash or a nonrash area.
Other viruses or allergies can cause a rubella-like illness, and there has been confusion and controversy over the clinical diagnosis of rubella for more than 100 years.103,104 Serologic studies have shown the lack of reliability of the clinical diagnosis of rubella by a physician or of the patient’s history of having had rubella. This is even more likely to be true today, when fewer and fewer physicians have personal experience caring for patients with the disease. Rubella virus infection also can occur without a rash.105 Therefore, it is essential to do specific serologic studies of a patient with the clinical diagnosis of rubella if the prevention of congenital rubella infection is involved.
Infection with wild-type rubella virus produces lifelong immunity against rubella virus-induced disease. In the prerubella vaccine era, when wild-type rubella virus was in wide circulation, asymptomatic reinfection with rubella virus, as defined by a rise in rubella antibody titer, was not unusual. Reinfection with a second clinical illness has been reported, although this presumably is exceedingly rare.106 There are no recent serologic surveys that examine the rate of asymptomatic rubella virus reinfection, but it would presumably be much less prevalent than it was in years past.
Arthritis is an occasional complication of rubella, especially in women. Testicular pain is not uncommon in postpubertal males.107 Other complications, such as encephalitis, are rare.108,109
Rubella immunization and congenital rubella syndrome are discussed in Chapter 19.
As noted, many viruses other than rubella can produce a rubella-like illness. Postauricular and occipital node enlargement have, in the past, been regarded as very useful in the diagnosis of rubella. However, this clinical pattern can be duplicated by infection with adenoviruses, echoviruses, or coxsackieviruses.103
Febrile Maculopapular Exanthem
This is a noncommittal descriptive diagnosis that can be made when the patient has fever, a maculopapular rash, and none of the typical associated findings of measles, roseola, or rubella.110,111 Typically, such illnesses occur more frequently in the summer and in outbreaks involving enough patients to make the physician recognize that this is the rash disease that is “going around.” Coxsackie viruses are the best-recognized causes of this type of rash and should be suspected when the rash appears within about 24 hours of the fever.112 Erythema infectiosum also may produce this type of rash but typically has little or no fever (see Fig. 11-2B, C).
Drug Rashes
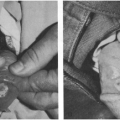
Drugs, particularly ampicillin, can cause maculopapular exanthems (Fig. 11-4). Often, the patient is receiving ampicillin for a febrile illness, so that the rash appears to be a febrile exanthem.
Other Causes
In 1951, a maculopapular exanthem that occurred after the patient’s fever had subsided and principally involved the face was observed in Boston. For
a time, the phrase “Boston exanthem” was used to describe this particular pattern. This Boston outbreak was caused by type 16 echovirus.111,113,114 Other outbreaks of exanthems occurred in Boston in 1959 and 1961 and were reported to be caused by different echovirus types or by coxsackieviruses and had a somewhat different clinical pattern. Based on this experience, some physicians felt that they could differentiate the viral etiology of an exanthematous disease based on the clinical pattern and rash distribution alone. However, there was always a fair amount of variability even in the patterns observed in a single outbreak. Thus, it is difficult if not impossible to predict the type of echovirus or coxsackievirus on clinical grounds.
a time, the phrase “Boston exanthem” was used to describe this particular pattern. This Boston outbreak was caused by type 16 echovirus.111,113,114 Other outbreaks of exanthems occurred in Boston in 1959 and 1961 and were reported to be caused by different echovirus types or by coxsackieviruses and had a somewhat different clinical pattern. Based on this experience, some physicians felt that they could differentiate the viral etiology of an exanthematous disease based on the clinical pattern and rash distribution alone. However, there was always a fair amount of variability even in the patterns observed in a single outbreak. Thus, it is difficult if not impossible to predict the type of echovirus or coxsackievirus on clinical grounds.
 FIGURE 11-4 Ampicillin rash, a maculopapular rash often confused with viral exanthems (Photo from Dr. Norman Fost). |
Respiratory syncytial virus infection has rarely been associated with a maculopapular rash, along with the more usual lower respiratory disease.115
Meningococcal bacteremia is a rare cause of maculopapular rash with high fever. A blanching blotchy rash has often been noted before the appearance of petechiae and probably is a vascular phenomenon. Maculopapular rashes may also be seen in Rocky Mountain spotted fever and, especially, ehrlichiosis.
Scarlet fever should always be considered in patients with maculopapular exanthems, because the rash is sometimes atypical. Other signs usually associated with scarlet fever may not yet have appeared. Lyme disease is a possible cause of a maculopapular rash, although the rash is usually macular. As discussed earlier, KD is usually associated with a maculopapular rash.
Diagnostic Approach
Throat cultures to exclude Group A streptococcal pharyngitis may be indicated in some maculopapular exanthems because scarlet fever is sometimes atypical.
If rubella is suspected and an exposure of a pregnant woman is involved, serum should be obtained as soon as possible during the first week of the illness for use as an acute-phase serum. This specimen can be held until a second serum is obtained 2–3 weeks later (convalescent-phase serum). Testing of paired sera is especially important for laboratory confirmation of rubella virus infection, but it also can be used for demonstration of measles virus infection in difficult cases.
Viral cultures can be obtained for recognition of infections caused by adenoviruses, echoviruses, or coxsackie B viruses. Recovery of one of these viruses is sometimes useful, along with negative serologic studies for rubella, to demonstrate that a rubella-like illness was not caused by rubella virus.
Treatment
Antibiotic therapy is not indicated before a specific diagnosis such as streptococci pharyngitis is confirmed unless the patient appears so seriously ill as to suggest possible septicemia or otitis media, pneumonia, or a similar complication is present.
Petechial-Purpuric Rashes and Vasculitis
Petechial rashes have been given special importance because they may be caused by bacteremia, particularly meningococcemia (Box 11-5). Petechiae typically are circular flat lesions 1 mm or less in diameter. At first, they are pink but change over the course of 1–12 hours to dark red and then to purple or brown. Unlike other exanthems, petechiae do not blanch with pressure. Petechiae can be seen in normal children after compression of an arm by a tourniquet or a blood pressure cuff or on the chest, face, or arms after prolonged coughing, crying, sneezing, forceful vomiting, or other Valsalva maneuvers. Petechiae also can be a result of thrombocytopenia, which can be produced by several noninfectious diseases, but the patient is usually not febrile or acutely ill.
Prospective studies on children hospitalized with fever and petechiae have generally documented approximately an 7% rate of meningococcemia.116 Interestingly, a more recent, larger prospective trial that enrolled all children under age 18 who presented to the emergency department with a temperature over 38°C and petechiae found that only 8 (2%) of 411 had bacteremia or clinical sepsis.117 All studies to date have shown that an approximation of the risk of serious disease in children with fever and petechiae can be refined by clinical and laboratory parameters, especially “ill appearance” of the child, the location of the petechiae, whether or not a mechanical factor known to produce petechiae exists, and peripheral WBC counts. In the largest study, the lack of ill appearance had a negative predictive value of 1.0, as did a WBC count between 5000 and 15,000/mL. A prothrombin time less than 13.5 seconds, a partial thromboplastin time of less than 30 seconds, and
the absence of purpura had negative predictive values of 0.99.117 Both studies were in agreement that if the petechiae were located only above the nipple line, or if an obvious mechanical factor caused the petechiae, the risk of serious infection is minimal. The incidence of serious infection in children with fever who had petechiae only above the nipple line was zero in both studies (total n = 601).
the absence of purpura had negative predictive values of 0.99.117 Both studies were in agreement that if the petechiae were located only above the nipple line, or if an obvious mechanical factor caused the petechiae, the risk of serious infection is minimal. The incidence of serious infection in children with fever who had petechiae only above the nipple line was zero in both studies (total n = 601).
BOX 11-5 Causes of Petechial or Purpuric Rashes
| Common “Viral syndrome” (no specific etiologic agent recovered) Valsalva effect of cough or vomiting Trauma of blood pressure cuff or tourniquet Streptococcal pharyngitis with petechiae |
| Uncommon Meningococcemia Pneumococceal bacteremia H. influenzae bacteremia Rocky Mountain spotted fever Ehrlichiosis Toxic shock syndrome Infective endocarditis Bacterial septic emboli (S. aureus, Pseudomonas, gonococcus) Disseminated intravascular coagulation (any cause) Infectious mononuclosis Adenovirus Cytomegalovirus, especially in newborn Rubella virus Coxackie or echoviruses Parvovirus B19 (papular-purpuric gloves and socks syndrome) RSV Influenza or parainfluenza viruses HIV Anaphylactoid purpura Thrombocytopenia from noninfectious causes |
| Rare Murine typhus Disseminated histoplasmosis Brucellosis Salmonellosis Q fever Dengue hemorrhagic fever Brazillan purpuric fever (H. aegyptius infection) Hantavirus Collagen-vascular diseases |
The blood pressure should be obtained immediately in any febrile patient with a petechial rash (see Chapter 10).
Purpura resembles traumatic bruising. Generally speaking, children who present with fever and purpura are at higher risk of having serious bacterial infections than are children with fever and petechiae. In Mandl’s study, the sensitivity of the finding of a purpuric rash in predicting serious disease was 83%, and the positive predictive value was 0.31, despite the very low incidence of invasive disease in their cohort.117 Children presenting with purpura and sepsis generally have other physical findings and laboratory values suggestive of sepsis.
Noninfectious diseases with signs resembling purpura include cutaneous vasculitis. In children, a relatively common form of cutaneous vasculitis is called anaphylactoid purpura (Henoch-Schönlein purpura), which occurs predominately below the waist except in infants.118 Purpura in children may also result from immune thrombocytopenic purpura, hemolytic-uremic syndrome or thrombotic thrombocytopenic purpura, thrombocytopenia due to HIV or other infectious agents, or thrombocyte dysfunction secondary to drug therapy. Rarely, aspirin in therapeutic dosages has been reported to cause thrombocytopenia119; this condition is rarer still now that most children never receive aspirin therapy.
Thrombocytopenia occurs commonly during bacterial septicemia as part of the syndrome of disseminated intravascular coagulation (DIC). This syndrome can also be caused by severe viral, fungal, or parasitic infections and is discussed further in Chapter 10.
Possible Infectious Etiologies
Meningococcemia
This is the most important cause to be considered in a patient with a petechial or purpuric rash, because the disease is rapidly fatal if untreated. Patients with a purpuric rash often develop septic shock or DIC, as described in Chapter 10. Patients with a macular or petechial rash are likely to have a better prognosis than those with purpuric lesions.120 Meningeal signs may be present, but meningococcemia can occur without meningitis and carries a worse prognosis. Early in the course of meningococcemia, the rash may be absent. In order to detect the possibility of occult meningococcemia, careful measurement of blood pressure and serial
physical examinations are indicated in the child with high fever.
physical examinations are indicated in the child with high fever.
Haemophilus Influenzae
In a study of 129 children hospitalized for fever and petechiae, 13 (10%) had Neisseria meningitidis, 8 (6%) had H. influenzae type b (Hib), and the majority had a viral illness.121 Septicemia secondary to Hib is now a rarity in the United States because of the routine use of the conjugated Hib vaccine.
Management of contacts exposed to the meningococcus or H. influenzae is discussed in Chapter 21.
Streptococcal Pharyngitis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree