Imaging Goals
Goals of radiographic imaging vary in patients with malignancies depending upon whether the specific malignant diagnosis is already known and whether the imaging is performed to stage disease or to follow disease. Each type of malignancy has its own spectrum of findings on imaging studies, which will be covered in detail in subsequent chapters. In this chapter, generalized imaging principles will be reviewed, with some attention to new imaging techniques that may have greater application in diagnosis and follow-up of malignancies in the future. Nuclear medicine will be covered in a separate chapter. Lymphangiography, angiography, and myelography will not be covered specifically in this chapter, because they are used less frequently in current practice. Detailed information regarding these types of studies may be obtained in a variety of oncoradiologic texts ( ).
Imaging findings in cancer patients may be very nonspecific or very specific (see Fig. 2.1 ). In choosing a particular method for imaging cancer patients, the specificity and sensitivity of the imaging modality must be considered with particular reference to the type of malignancy suspected. The risk to the patient for the imaging modality must also be weighed, along with the actual cost of the study. The strengths and weaknesses of many common studies will be considered individually, with a discussion of imaging assistance for biopsy, which is often the final path to conclusive diagnosis of malignancy.
It is important even in cancer patients, who may have already received large doses of radiation as part of their treatment and who are considered to have life-threatening conditions, to keep in mind the principle of ALARA (as low as reasonably achievable), which guides efforts to minimize radiation exposure in imaging of all patients. Recent evidence suggests that overuse of medical imaging can lead to an increase in cancer risk, particularly in women and in young patients ( ). As treatments for cancer become more successful and patients live longer, the cumulative risk of follow-up imaging, particularly multidetector computed tomographic (CT) scans, must be considered in planning appropriate management.
Plain Radiography
Digital radiographic imaging has replaced analog (film-screen) imaging for most plain radiography. In computed radiography a phosphor-coated plate is substituted for the photographic film used in analog radiography. This plate is exposed using standard x-ray equipment and is then read out electronically in the form of digital imaging data. The plate can be erased and reused. The digital imaging data can be used to print images on transparent film or paper or displayed on a monitor. Digital imaging has many advantages over film-screen radiography, including fewer under- or overpenetrated films, ability to change contrast and brightness of the image after acquisition, existence of edge-enhancing algorithms for image processing, and digital storage, allowing electronic transfer of imaging information ( ). Drawbacks include slightly lower spatial resolution (not noticeable without special testing methods) and initial expense of installation. Combination of digital imaging systems with radiology scheduling and reporting systems allows progression to a “film-less” radiology department, using a totally integrated “picture archiving and communication system” or PACS ( ). Particularly with newer, faster computer systems and improvements in rate of data transfer, PACS systems have proliferated at most academic centers and have become the standard for large institutions throughout the country.
Plain radiographs are often the most cost-effective method to begin a diagnostic search for malignancy or to follow effects of treatment. Table 2.1 compares cost, radiation dose, and practical limitations for various plain radiographic examinations. Cost estimates are for a large U.S. tertiary-care teaching institution in New England and will vary in other types of institutions and other parts of the country. Dose information is given as skin entry dose, which is at best a crude estimate of actual absorbed dose. Specific absorbed doses to particularly sensitive organs should also be considered and will vary depending on the specific views obtained. For example, the breast glandular dose from an anteroposterior (AP) chest radiograph (wherein the beam enters the front of the patient) is about 70 times greater than the breast glandular dose from a posterior-anterior (PA) chest radiograph. Overall, approximately 50% of the average radiation dose to the general public is from radon gas and only about 15% from medical imaging, including nuclear medicine ( ).
| Exam | Cost | Radiation Dose † |
|---|---|---|
| PA/lat CXR | 1 cost unit * | 0.007–0.01 cG (PA) |
| 0.02–0.03 cG (lat) | ||
| Portable AP CXR | 1.25 | 0.01–0.02 cG |
| KUB | 0.5 | 0.06–0.1 cG |
| Decubitus abdomen | 0.5 | 0.06–0.1 cG |
| AP & lat C-spine | 1 | 0.06 cG (AP) |
| 0.05 cG (lat) | ||
| AP & lat T-spine | 1 | 0.2 cG (AP) |
| 0.3–0.6 cG (lat) | ||
| AP & lat L-spine | 1 | 0.07–0.1 cG (AP) |
| 0.2–0.3 cG (lat) | ||
| Femur, humerus | 0.75 | 0.08–0.2 cG each view |
| Pelvis | 0.85 | 0.05–0.06 cG |
| Ribs | 0.75 | 0.15 cG (two views) |
| Knee, shoulder, hip | 1 | 0.07–0.1 cG each view |
| AP & lat skull | 1 | 0.1–0.3 cG (AP) |
| 0.1–0.3 cG (lat) | ||
| Barium swallow | 1.4 | 3–5 cG |
| UGI (air-contrast) | 2.25 | 8–15 cG |
| UGI/SBFT | 2.75 | 10–25 cG |
| SBFT | 2.25 | 3–5 cG |
| Enteroclysis | 3 | 10–15 cG |
| BE (air-contrast) | 3.1 | 15–30 cG |
| Mammogram | 2 | 3 mG or less film-screen, per view |
| ~2 mG or less digital, per view | ||
| Head CT | 3.25 (1−) | 4–6 cG |
| 3.75 (1+) | ||
| 4 (1−/1+) | ||
| Chest or abdomen CT | 3.6 (1−) | 1–3 cG |
| 3.9 (1+) | ||
| 4.25 (1−/1+) | ||
| Pelvis CT | 3.25 (1−) | 1–3 cG |
| 3.75 (1+) | ||
| 4 (1−/1+) |
* One cost unit, for comparison purposes, is defined as the cost for a PA and lateral CXR, including both technical and professional fees.
† Doses are given as skin entry doses; actual absorbed dose will vary considerably depending on radiographic technique, body habitus, and site examined. These doses also assume use of state-of-the-art image receptors and optimal radiographic technique.
CHEST RADIOGRAPHS
The chest radiograph provides an excellent survey of the lungs, mediastinum, bony thorax, and pleura in a very short exam time and at a relatively low radiation dose. Because of the natural contrast between air in the lungs and soft tissue in other parts of the chest, the chest radiograph is particularly sensitive for diagnosis of many pulmonary malignancies, though usually not very specific. Chest radiographs may also provide useful staging information for lung tumors in some cases, because bone metastases and mediastinal adenopathy may be demonstrated along with the primary tumor. Chest radiographs are also useful in detection of many treatment-related problems, such as infections, drug toxicities, fluid overload, and misplaced support lines. Problems with support lines are one of the most common abnormalities detected on portable radiographs. Even a subtle abnormality may have clinical importance. A finding of an abnormally placed line on a chest radiograph may require additional studies, such as CT or digital subtraction angiography, to determine the exact line position and whether it can be used for infusion of chemotherapy.
Chest radiographs in obese patients will show decreased contrast, but even in patients in excess of 160 kg (352 lb), diagnostically useful radiographs can usually still be obtained. There is an overall decrease in diagnostic quality of many types of imaging studies in obese patients, but in the cancer setting this is less of a problem than in the general population. For solving specific problems in the chest, special views may be useful, such as oblique views (helpful for detection of rib, pleural, or chest wall lesions and for confirming the presence of questionable lung nodules), decubitus views (for demonstrating loculation of pleural fluid and for improving visualization of the lung base in the presence of large nonloculated pleural effusions), apical lordotic views (for projection of the apical portion of the lung free of overlying bony structures), or apical kyphotic views (for examination of the pleural apex). Chest radiography can be performed at the bedside; however, the quality of these exams is limited by certain fixed technical factors. The portable x-ray machine does not generate the same kilovoltage (kVp) as a standard chest unit (60–90 kVp vs 120 kVp for standard chest radiography). Also, the distance from the tube to the receptor is shorter than for departmental radiographs (0.9–1.2 m vs 1.8 m for standard chest radiography), which increases distortion and geometric indistinctness. Portable radiography may also be limited by patient condition and difficulty in properly positioning the receptor and x-ray tube.
ABDOMINAL RADIOGRAPHS
Plain radiographs of the abdomen provide a less sensitive diagnostic study for abdominal organs than the chest radiograph provides for the lung, because the air in the bowel provides the only natural contrast among the abdominal contents. Detection of retroperitoneal and solid-organ abnormalities is limited, unless calcification is present. Fat planes within the abdomen may provide enough contrast to allow delineation of some solid-organ contours but is variable among patients. Fat planes may be particularly diminished in cancer patients, because of weight loss related to their disease or their treatment. However, for detection of abnormalities of the bowel, plain abdominal radiographs can be very helpful and again, are inexpensive, can be obtained at a relatively low radiation dose, can be performed at the bedside, and require only minimal patient cooperation. Special views that may be helpful include decubitus views (left side down for detection of minimal pneumoperitoneum outlined by the liver), prone views (to move gas into the rectum and rule out distal colonic obstruction), and upright views (to detect air-fluid levels, which are only normal in the stomach and duodenal bulb, but can be seen in the colon in patients with diarrhea or in the small bowel in cases of ileus or obstruction). Upright abdominal radiographs can also be used to detect pneumoperitoneum, but the upright chest radiograph is preferable because the x-ray beam is centered closer to the dome of the diaphragm and therefore will more clearly demonstrate very small collections of air. Abdominal fluoroscopy is not usually performed without contrast administration.
BONE RADIOGRAPHS
Bone plain radiographs are moderately sensitive in detection of many primary and metastatic malignancies but are most useful when interpreted in conjunction with results of nuclear medicine bone scans. Interpretation of bone radiographs can be confounded by a variety of normal variants and benign lesions and experience in interpretation of bone radiographs is essential. In surveying the body for bone metastases, nuclear medicine scanning is preferred to skeletal surveys, because the bone scan is more sensitive, less expensive, and gives a lower radiation dose. The exception to this rule is in multiple myeloma or in very aggressive purely lytic bone metastases, wherein bone scanning may be negative ( ). In these cases, skeletal surveys or plain radiographs of long bones, skull, spine, and pelvis are preferred. Consultation with a radiologist may often be helpful in limiting any bone examination to the most appropriate radiographs. For certain bones, such as the sacrum, scapula, and sternum, CT or tomography is required for best visualization of the entire bone. The particular views included in a standard study of any bone or joint will vary from department to department. Therefore, if a specific question is to be answered regarding a bone or joint, adequate clinical information must be given to the radiologist to decide if additional views must be obtained to supplement the standard views.
Gastrointestinal Contrast Studies
The role of gastrointestinal (GI) contrast studies has become progressively more limited, with many of the indications for theses studies being replaced by CT or magnetic resonance imaging (MRI). However, for direct examination of dynamic peristaltic function or for detection of perforation, these studies still can have a role. Care must be taken in planning the sequence of GI studies when staging a cancer patient, particularly if contrast studies are needed. If a barium enema, bone scan, upper GI series, and CT are all planned, the CT or bone scan should usually be done first (in that barium from the other two studies will severely limit the ability to perform the CT or nuclear medicine studies for varying lengths of time, up to a week). The barium enema should then be performed before the upper GI series, because residual contrast will hamper either study, and contrast from a barium enema is usually eliminated more rapidly than that from an upper GI study. When in doubt about how best to schedule a series of different types of radiologic studies, consultation with a radiologist or nuclear medicine physician is often helpful.
Intravenous Contrast Studies
Intravenous (IV) contrast agents used in radiology are now almost entirely iso-osmolar to blood (non-ionic agents), having replaced older ionic agents that were less expensive but more toxic. The incidence of fatal contrast reactions with either type of agent is approximately 1/100,000 uses ( ). The nephrotoxic effect of non-ionic agents is probably less than with older ionic agents, and the incidence of minor contrast reactions (nausea, vomiting, hives) is definitely much lower with non-ionic agents, which leads to much better patient acceptance. All contrast agents must be used with caution in patients with multiple myeloma, diabetes, sickle cell disease, or chronic renal insufficiency.
In patients with a history of serious reactions to IV contrast material, a premedication protocol using steroids and histamine blockers is often used before a planned contrast administration ( ). Whenever possible, IV contrast should be administered after the patient has taken nothing by mouth for several hours, because food or liquids in the stomach may increase the risk of vomiting. Because venous access is often a problem in cancer patients, placement of an IV catheter before a planned study is often helpful to prevent delays or cancellation of the study. In CT, optimal examinations require rapid bolus contrast administration. Therefore, the largest-caliber catheter that can be easily inserted should usually be used. Contrast material that leaks from a small-caliber catheter into the surrounding tissues (infiltration) can lead to considerable pain, swelling, and even tissue necrosis. With newer multidetector CT (MDCT) equipment, which is capable of completing the scan of a region such as the liver in a very short time, it is now possible to reliably obtain image sequences at various phases after injection to optimize visualization of lesions such as metastases ( ).
INTRAVENOUS AND RETROGRADE UROGRAPHY
The advent of CT and ultrasound has markedly decreased indications for IV and retrograde urography. In an IV pyelogram, after injection of a bolus of IV contrast material, radiographs and tomograms are obtained rapidly to demonstrate the enhancement of the renal parenchyma, followed by more delayed radiographs to show the contour of the collecting systems, ureters, and bladder. Filling of the collecting systems is highly variable, and it is not uncommon for small segments of the ureters to be poorly visualized. If small mucosal lesions of the collecting system are suspected, retrograde studies may be preferable. Cystography involves filling the bladder with contrast via catheter, usually under fluoroscopic guidance. To visualize the urethra, the catheter can be withdrawn after filling of the bladder and radiographs obtained during urination in a voiding cystourethrogram (VCUG). In conjunction with cystoscopy, the ureters can also be cannulated and retrograde injections may be performed into the collecting systems under fluoroscopic guidance. This provides the best visualization of the entire collecting system. The cost for an IV pyelogram is approximately 3.25 cost units, and the radiation dose is 3–6 cG. The cost of a cystogram is approximately 1.5 cost units, a VCUG is 2.25 cost units, and a retrograde CUG is 2.5–3 cost units. The radiation dose is 3–6 cG, with particularly high gonadal doses for the VCUG.
Mammography
The quality of imaging in mammography has improved rapidly in recent years. The two goals of maximizing spatial and contrast resolution and minimizing patient dose have led to many technical innovations. Early mammography was performed with standard radiographic equipment and one view of each breast. Current mammographic standards require dedicated mammographic equipment, strict quality control standards, and two views of each breast, in the craniocaudal and mediolateral oblique projections. Specialized views may also be obtained, such as spot compression (to search for nodules or architectural distortion), rolled or rotated (to help localize a lesion within the breast), or magnification views (to detect and characterize microcalcifications). When a mass is present that may represent a cyst, breast ultrasound may be useful. In recent years most large centers have switched from film-screen to digital mammography ( Fig. 2.2 ). Mammography was the last radiographic imaging modality to convert to a digital format because of the extremely high spatial resolution required for diagnostic images. New receptors had to be developed as well as a new generation of high-resolution high-brightness viewing monitors before digital mammography became a practical reality. Most studies have shown similar sensitivity and specificity of digital and film-screen mammography except in the setting of dense breast tissue, where digital mammography is probably slightly more sensitive ( ). Another advantage of digital mammography is the ability to apply sophisticated computer-aided diagnostic techniques to the images to improve sensitivity and specificity. The overall breast radiation dose for digital mammography is slightly lower than for conventional film-screen imaging if imaging parameters are optimized ( ).
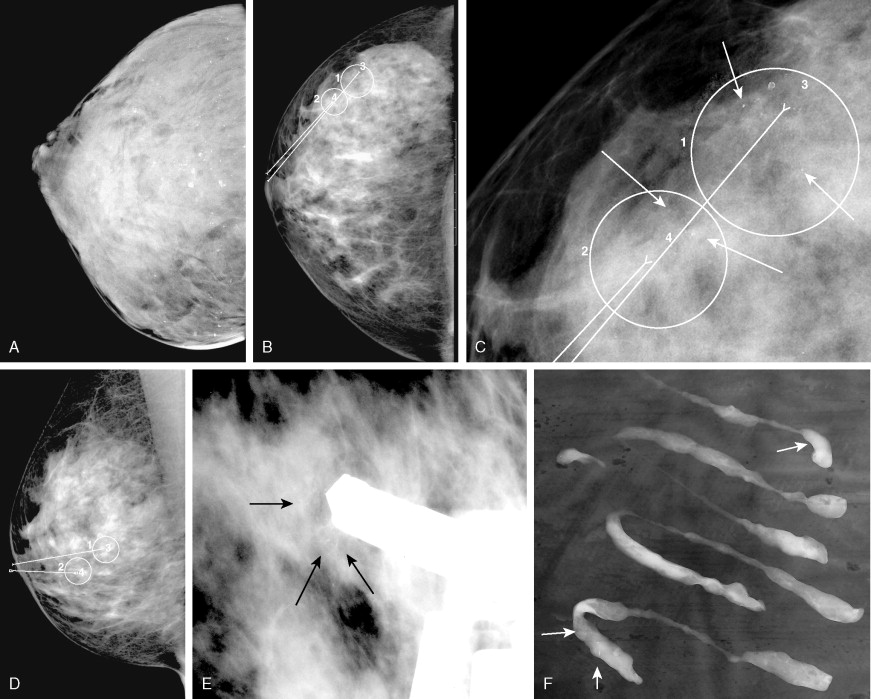
There are a number of interesting new technical adaptations of mammography on the horizon that may greatly increase our ability to detect small early breast tumors. These include breast tomosynthesis (which creates a three-dimensional [3D] view of the breast) and contrast-enhanced mammography (which may image neovascularity in tumors) ( ). The mean glandular dose from modern film-screen or digital mammography is under 0.3 cG for each view, which would yield approximately 10/1,000,000 excess cases of breast cancer in 50-year-old patients due to the radiation from a standard four-view examination ( ). This produces a risk of dying from a radiation-induced breast carcinoma comparable to the risk of dying due to smoking 14 cigarettes, breathing the air of an industrialized city in the U.S. Northeast for a month, or living in Denver for 2 years ( ). Mammographic dose varies with breast size, density, degree of compression, and type of x-ray target used, and radiation risk from mammography decreases with increasing patient age.
It is very important when ordering a mammogram that any prior mammograms be available to the radiologist at the time of the examination. Having prior mammographic reports is not sufficient, and actual images are needed to optimize the search for subtle changes in density and architecture. Comparison with prior studies greatly decreases the need for extra views and other workup, such as ultrasound, also greatly decreasing patient anxiety. Findings on mammography generally can be grouped into categories based on the likelihood of malignancy, with varying follow-up recommendations based on this classification ( Table 2.2 ). A normal mammogram does not exclude malignancy, because the false-negative rate of mammography ranges from 10% to 20% ( ). Mammography may be uncomfortable for the patient, because firm compression of the breast is needed for best diagnostic image quality ( ). Patients are instructed not to use deodorant on the day of their exam, because it may be visible on the images and can mimic pathology (microcalcifications). If patients experience cyclic changes in sensitivity of their breasts, it is helpful to schedule their mammogram at a time when they anticipate the least sensitivity. Mammograms are not generally recommended for women under the age of 30, but with newer equipment, adequate studies may be obtained even in young women if the clinical situation is sufficiently worrisome. For example, in patients who received mediastinal radiation at a young age for treatment of Hodgkin’s lymphoma, routine yearly mammography should generally begin approximately 10–15 years after completion of the radiation, which in many patients will be at an age well under 30 (Diller et al., 2003).
| Finding | Significance (BI-RADS * category) | Follow-up | Additional Procedures |
|---|---|---|---|
| Vascular calcification | Benign (BI-RADS 2) | Routine † | None |
| Skin calcification | Benign (BI-RADS 2) | Routine | Tangential radiographs |
| Simple cysts | Benign (BI-RADS 2) | Routine | Aspirate, if painful |
| Intramammary lymph nodes | Benign (BI-RADS 2) | Routine | Ultrasound, to rule out cyst, detect fatty hilum of node |
| Complex cyst | Indeterminate (BI-RADS 4) | Depends on results of aspiration | Aspirate or excise |
| Multiple bilateral clusters of microcalcifications | Indeterminate (BI-RADS 3 or 4) | 6-month f/up | Needle localization, if any one group is more worrisome in morphology |
| Solid, smoothly marginated nodule on initial study | Indeterminate (BI-RADS 3 or 4) | 6-month f/up | Consider core biopsy or excision; ultrasound to rule out cyst |
| Multiple nodules | Indeterminate (BI-RADS 3) | 6-month f/up | Consider core biopsy or excision if any are irregular in outline |
| Asymmetrical parenchymal densities | Indeterminate (BI-RADS 3) | 6-month f/up | Spot compression radiographs to exclude underlying architectural distortion |
| Single cluster of microcalcifications | Possibly malignant (BI-RADS 4) | Depends on results of biopsy | Biopsy, with needle localization |
| New solid nodule | Possibly malignant (BI-RADS 4) | Depends on results of biopsy | Biopsy, with needle localization if not palpable |
| Architectural distortion | Possibly malignant (BI-RADS 4 or 5) | Depends on results of biopsy | Biopsy, with needle localization if not palpable |
| Spiculated mass | Probably malignant (BI-RADS 5) | Depends on results of biopsy | Biopsy, with needle localization if not palpable |
* BI-RADS (Breast Imaging-Reporting and Data System) categories based on American College of Radiology quality assurance program for breast imaging.
† Routine mammographic follow-up, as recommended by the American College of Radiology, consists of yearly or biennial mammography for women between the ages of 40 and 49 and yearly mammography for women aged 50 or older.
If the clinician feels a palpable lesion that is to be evaluated, the exact location of that lesion should be clearly communicated to the radiologist and may ideally be marked on the skin to further help in planning the examination. Mammography in people with breast implants is more complicated and requires a total of four views of each breast in most cases ( ). Breast size does not alter the technical ability to perform an adequate mammogram, and high-quality images can be obtained in small-breasted women as well as in most men. Patient immobility can compromise the examination, because this may limit the ability of the technologist to bring the entire breast into the x-ray field. Other features of oncologic patients that may compromise mammography include massive ascites; recent breast, axillary, or chest wall surgery; and implanted reservoir catheters overlying the upper breast.
Ultrasound
Ultrasound offers many advantages as an imaging modality for the cancer patient. The study is generally painless and can be performed rapidly at the bedside. Ultrasound does not involve ionizing radiation, and no oral or IV contrast materials are generally needed. The study is particularly attractive for frequent follow-up examinations for these reasons. Ultrasound can be used as a guide for biopsy or for drainage of pleural, pericardial, or peritoneal fluid. Using intracavity probes, very detailed images of pelvic organs can be obtained, as well as biopsies. Ultrasound can be combined with endoscopy for examination of the heart or esophagus ( ) and can also be performed intraoperatively to assist in accurate tumor localization ( ). With color flow and Doppler capability, venous thrombosis can be detected noninvasively. Vascular imaging of the upper extremity with ultrasound is more difficult than the lower extremity because of the sound-dampening qualities of the bony thorax and clavicle. In any ultrasound examination, an acoustic “window” is needed to allow the sound beam to pass into the area to be examined. Bone and air do not transmit sound waves, and therefore ultrasound of the chest is limited to the heart (which can be approached through the mediastinal tissues just lateral to the sternum for evaluation of cardiac chamber size, wall motion, and pericardial fluid) and pleural fluid collections that touch the inner chest wall. The cost of ultrasound examinations ranges from 1.75 cost units for a breast ultrasound to 3.25 cost units for bilateral lower extremity venous ultrasounds.
BREAST ULTRASOUND
Breast ultrasound may be a helpful adjunct to mammography but is not useful as a screening tool ( ). Ultrasound can demonstrate the cystic quality of some breast lesions, eliminating the need for further workup. The exam is very operator-dependent, and images may be difficult to reproduce because of variable transducer position and settings from one exam to the next. Therefore, breast ultrasound is best used in evaluating specific lesions, such as nodules visible on mammography or palpable lesions. Ultrasound may be used as a guide for cyst aspiration, which can be both diagnostic and therapeutic. Ultrasound-guided core biopsy of the breast has greatly improved our ability to quickly assess for possible recurrences in breast cancer patients, and may even be able to locate and diagnose sentinel nodes before definitive surgery ( ). Ultrasound is also useful in detection of rupture of breast implants. Future directions for breast ultrasound include detailed Doppler analysis of blood flow in the region of possible tumors as an indicator of neovascularity ( ).
ABDOMINAL ULTRASOUND
Abdominal ultrasound in the cancer patient may detect liver metastases, dilated bile ducts, hydronephrosis, and masses. Some liver metastases may be better seen with ultrasound than with CT. Measurement of liver and spleen size can be obtained but may be difficult to reproduce because of the relatively small field of view of the ultrasound beam. Measurements are particularly difficult in patients with marked organomegaly, which moves the borders of the organ beyond the range of the transducer, requiring multiple images to encompass the entire organ. Ultrasound is very sensitive in detection of ascites and may be useful in guiding paracentesis. It can also be used to guide percutaneous biopsies of abdominal lesions and for placement of nephrostomy tubes. Evaluation of the pancreas can be limited in some patients by gas in the stomach and duodenum, which blocks sound waves. Ultrasound of the abdomen may also be limited in very obese patients, because the transducers have fixed depths of penetration and fat is relatively attenuating to the sound beam. The presence of barium in the GI tract can severely limit abdominal ultrasound, because the barium blocks sound. Only minimum patient cooperation is required for most abdominal ultrasound examinations, which can be performed at the bedside.
PELVIC ULTRASOUND
Pelvic ultrasound is generally useful to detect small amounts of ascites or to detect tumors of the pelvic organs. For examination of the uterus and ovaries, either a transabdominal or transvaginal approach may be used. Many patients prefer the transvaginal approach, because the transabdominal approach requires pressing the transducer against a full bladder to provide an acoustic “window” onto the pelvis. For prostate examination, similarly, a transabdominal or transrectal approach may be used. More patient cooperation is required for transvaginal or transrectal ultrasound than for the abdominal approach, which can be performed at the bedside. Biopsies can be performed using special needle guides on the rectal and vaginal probes. As in the abdomen, obesity can limit imaging using the transabdominal approach.
ULTRASOUND ABLATION
The ultrasound beam can be focused into a small area with special electronics to produce tissue heating at a single point or plane, called high-intensity focused ultrasound. Any lesion that can be visualized with routine ultrasound is a potential target for ultrasound ablation, which has been performed in the prostate ( ), for uterine fibroids ( ), and in many other solid-organ tumors such as liver, kidney, and pancreas ( ). High-intensity focused ultrasound has a great advantage over other ablation techniques in that it does not require insertion of a catheter into the organ to be treated, because probes can be placed on the skin surface and treatments may require only light sedation.
Computed Tomography
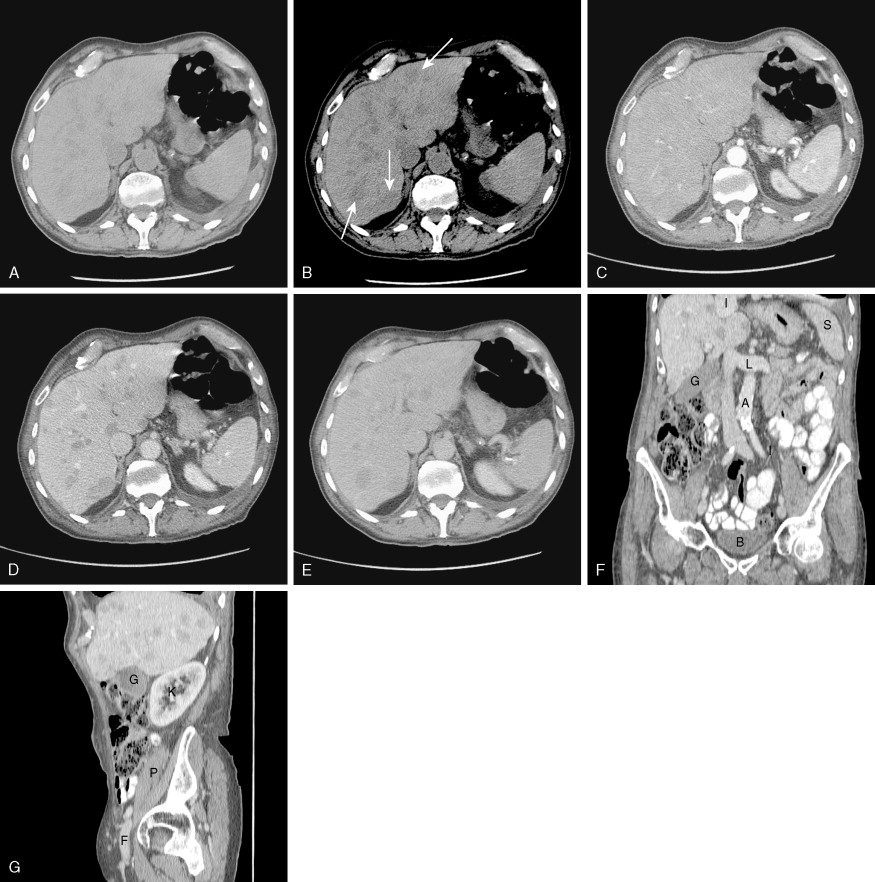
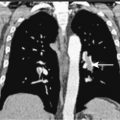
CT scanners have undergone a technical revolution since the introduction of the first helical scanners. Helical scanning, with continuous rotation of the radiation source and continuous feeding of the patient through the scanning gantry, offers much shorter scan times than older single-slice units. The most recent technical development in CT is the development of MDCT scanners, which are units containing several rows of detectors that can simultaneously detect photons while the patient passes through the imaging field, generating much more data in an even shorter time interval. Initial (MDCT) units contained 8 rows of detectors, but current units contain 64 and even more will be available in the future. CT offers many advantages over other imaging methods, including accurate, reproducible measurement of tumors, detection of bone metastases, and detection of enlarged lymph nodes ( ). MDCT, with its vast amount of data, allows sophisticated 3D applications to become a practical reality. In CT colonography, even quite small mucosal lesions can be seen ( Fig. 2.3 ), and automated volumetric measurements are now possible for even small lung nodules, which should allow greatly improved assessment of treatment response ( ). CT is relatively expensive, with many examinations costing over 3.5 cost units. Examinations of contiguous portions of the body require separate exams, so that the bill for a head, chest, abdomen, and pelvis study can total over 15 times the cost of a PA and lateral chest exam. The cost is even higher if IV contrast is used. However, for many areas of the body, such as the abdomen or mediastinum, no other imaging modality offers such complete information.
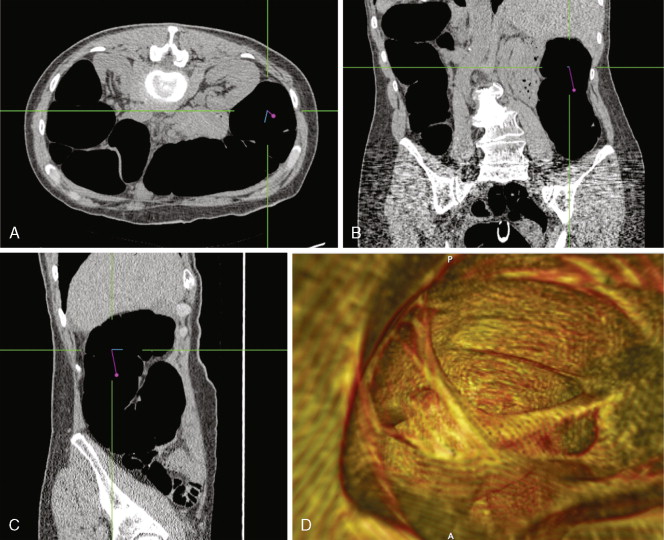
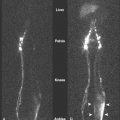
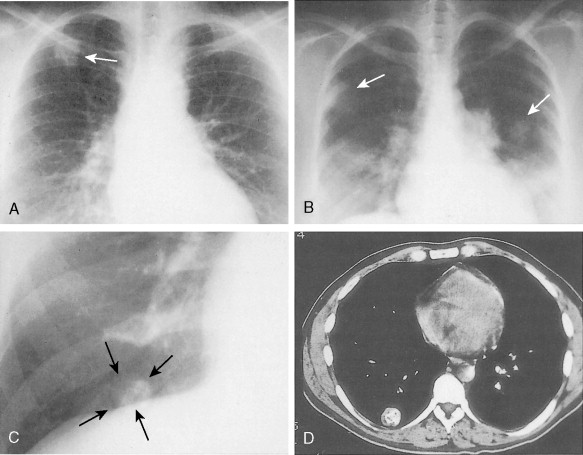
In the past, helical or spiral CT scanning required several minutes to complete for a study encompassing the chest, abdomen, and pelvis. This limited the thickness of slices that could reasonably be obtained without patient movement, and prevented routine multiplanar reconstruction of data. However, with the introduction of MDCT scanners, which are capable of scanning extremely quickly over large areas of the body, it is now routine to obtain slices thinner than 1 mm, which can easily be reconstructed in sagittal and coronal planes, as well as in 3D volumetric reconstructions ( Fig. 2.4 ). Such sophisticated multiplanar methods allow much more accurate determination of tumor volumes and response rates to be routinely calculated. The downside of these technical developments is the increase in total numbers of images that must be interpreted in each CT study, but application of computer-assisted diagnostic methods will provide assistance with these image management issues in the future ( ).