Malignant mesothelioma is a rare malignancy arising from the mesothelial cells of the pleural or peritoneal surfaces. Mesothelioma can arise from the pleura (pleural mesothelioma), peritoneum (peritoneal mesothelioma), pericardium (pericardial mesothelioma), or tunica vaginalis (testicular mesothelioma). In the United States there are approximately 3000 new cases of mesothelioma reported annually. Approximately 80% of these occur as pleural mesothelioma.
The development of mesothelioma is most clearly associated with prior asbestos exposure ( ). Asbestos was (and continues to be in some parts of the world) an important and affordable industrial resource as a result of its resistance to heat and combustion. Asbestos was used in shipbuilding, in car brakes, in the production of cement, and as insulation. There are two main forms of asbestos known as amphiboles and chrysotile. Amphiboles are long, thin asbestos fibers and are believed to be the most carcinogenic of the asbestos fibers. Chrysotile asbestos has also been associated with mesothelioma, although the frequency may be less than with amphibole asbestos ( ). The latency period between the time of asbestos exposure to development of mesothelioma can be 20–40 years. These unique features reflect the population of patients who develop mesothelioma including asbestos miners, plumbers, pipefitters, or those who worked in shipbuilding industries. In the United States mesothelioma is a disease of Caucasian men, reflecting the population of asbestos workers in the 1960s and early 1970s. The median age of patients diagnosed with mesothelioma is the mid-60s, although in the Surveillance Epidemiology and End Results database from the United States the median age is over 70. Approximately 80% of patients who develop mesothelioma are men. Women who develop mesothelioma also may have worked in industries that used asbestos, although there are reports of secondary exposure, for example, from clothing of spouses who worked directly with asbestos ( ). The estimated incidence of mesothelioma worldwide also reflects the use of asbestos in different regions of the world. A second, but very controversial factor thought to have a role in the development of mesothelioma is simian virus 40 (SV40). SV40 is an oncogenic polyomavirus in human cells, and infection with it leads to inactivation of tumor suppressor genes TP53 and the retinoblastoma gene ( RB1 ) ( ). SV40 may have been transmitted to humans inadvertently as a contaminant in the polio vaccine 30–40 years ago. However, epidemiologic studies have not found a greater incidence of mesothelioma in those who received the polio vaccine during that time period ( ). Although SV40 viral sequences have been detected in mesotheliomas (and not in normal adjacent lung tissues), this has not been a consistent finding and, as suggested by some investigators, may even be a false-positive finding ( ). Other etiologic factors leading to mesothelioma include prior ionizing radiation and rare familial forms reported to occur in Cappadocia, Turkey ( ).
Clinical Presentation
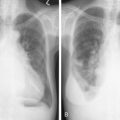
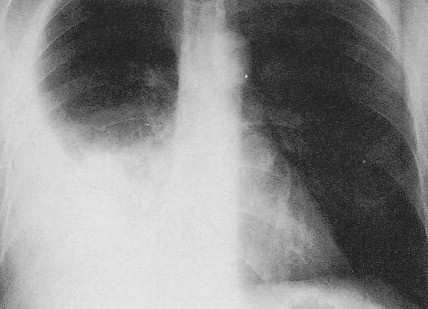
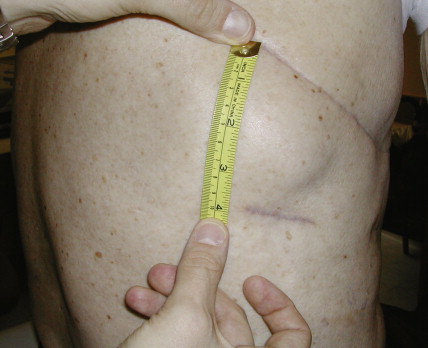
The most common clinical presentation of malignant pleural mesothelioma includes dyspnea on exertion and shortness of breath. These clinical symptoms often lead physicians to obtain a chest radiograph wherein a unilateral pleural effusion is noted ( Fig. 6.1 ). Mesothelioma is rarely an incidental finding on a routine chest radiograph. Patients can also present with nonpleuritic chest pain. This symptom is important to elicit from patients, because those who present with chest pains often have disease extending into the chest wall and thus are not surgical candidates. Other presenting signs and symptoms include discordant chest wall expansion, weight loss, night sweats, and the presence of a palpable subcutaneous mass ( Fig. 6.2 ).
Diagnostic Evaluation
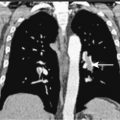
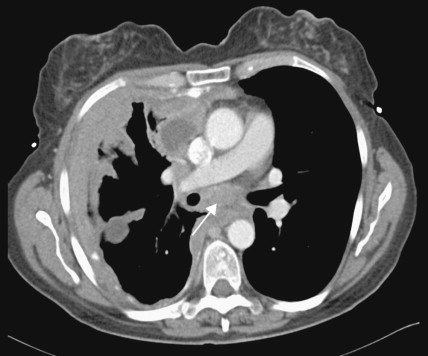
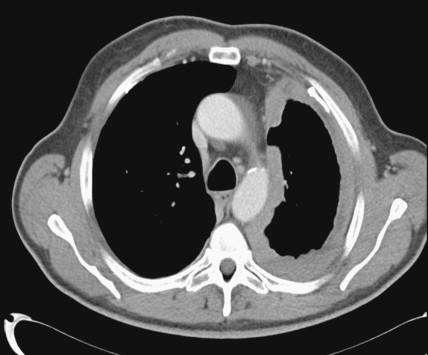
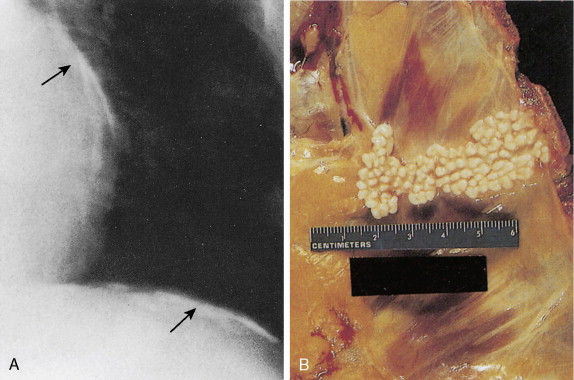
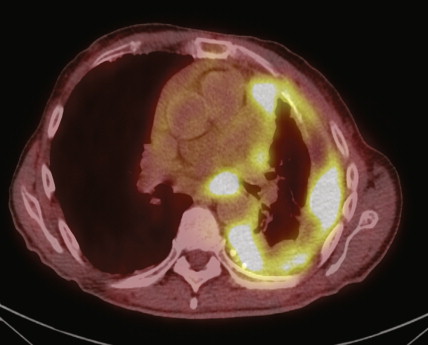
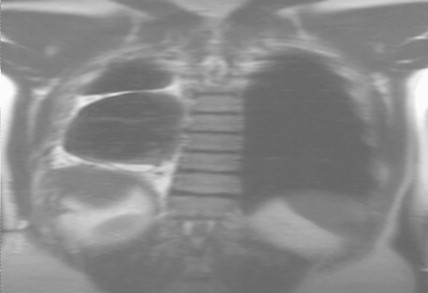
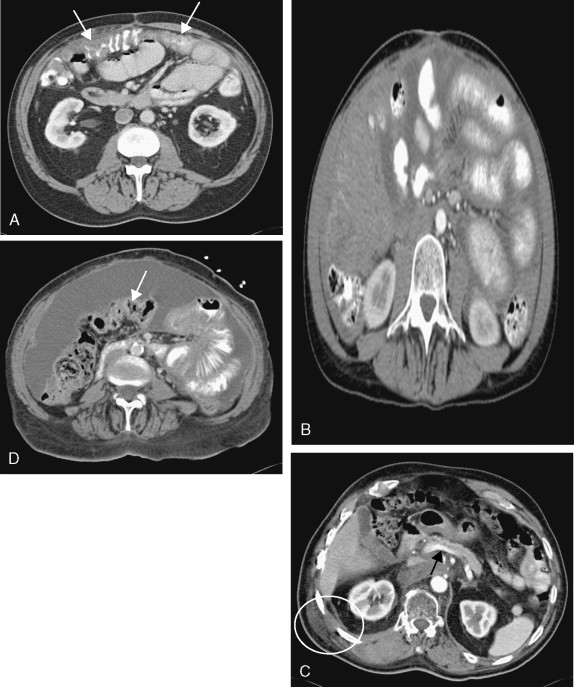
Three main imaging modalities, computed tomography (CT), positron emission tomography (PET), and magnetic resonance imaging (MRI), are used to evaluate patients with newly diagnosed mesothelioma. Contrast-enhanced CT scanning often reveals a thickened lobulated circumferential pleural rind ( Figs. 6.3 , 6.4 ) with or without the presence of a concurrent pleural effusion. CT scanning can also help identify the presence of pleural plaques ( Fig. 6.5 ), a sign of previous asbestos exposure, and whether the disease extends into the interlobar fissures. In addition, a contrast-enhanced CT scan can help determine the presence of mediastinal lymph nodes ( Fig. 6.3 ). 2-[ 18 F]-fluoro-2-deoxy- d -glucose (FDG)-PET scanning has also recently been evaluated for its clinical utility, in that most mesotheliomas are PET-avid ( Fig. 6.6 ). Analogous to lung cancer, FDG-PET scanning can identify occult metastatic disease in patients who are otherwise potential surgical candidates ( ). However, its utility in defining locoregional disease and the role of FDG-PET scanning in advanced mesothelioma remain to be determined. MRI can also be useful in the evaluation of mesothelioma, because it can identify chest wall or transdiaphragmatic invasion and involvement of the intralobular fissures ( Fig. 6.7 ). Peritoneal mesothelioma is more difficult to image accurately. CT scanning often reveals a thickened omentum and/or the presence of ascites ( Fig. 6.8A to C ). FDG-PET scanning has also been used in the evaluation of peritoneal mesothelioma. The peritoneal cavity is also a common site of mesothelioma recurrence in patients who have undergone prior surgical resection of their thoracic disease ( Fig. 6.8D ).
The presentation of a patient with a new pleural effusion often leads to a thoracentesis to evaluate the nature of the effusion. The pleural effusions in mesothelioma are often cytologically negative, and patients frequently undergo repeated thoracenteses to establish the diagnosis of mesothelioma or before performing a more invasive evaluation. Because of the frequent nature of cytologically negative effusions, cytology is not a reliable method of diagnosing mesothelioma. In addition, cytologic specimens are often insufficient to determine the histologic subtype of mesothelioma, which itself provides prognostic information. The diagnostic procedure of choice for mesothelioma is a thoracoscopic biopsy. There are several advantages to this approach. First, it provides the surgeon the ability to visualize the extent of involvement of the mesothelioma to determine whether it is located on the parietal and/or visceral pleural surfaces. Second, a biopsy specimen of the involved area can be obtained under direct visualization. This is critical for making an accurate histologic diagnosis. Finally, thoracoscopy provides the ability to also perform a pleurodesis, which can help control recurrent pleural effusions.
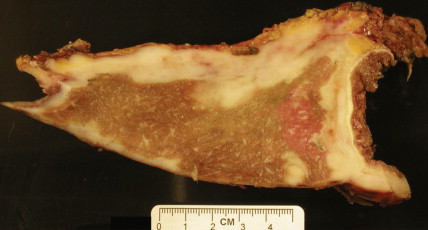
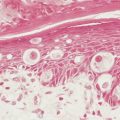
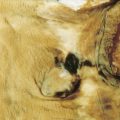
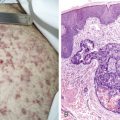
On gross pathologic evaluation mesothelioma can be seen as a pale infiltrative tumor that encases the lung ( Fig. 6.9 ). It can also infiltrate the underlying lung parenchyma and/or involve local and regional lymph nodes ( Fig. 6.10 ). The most common histologic subtype is epithelioid mesothelioma and accounts for approximately 60% of all mesotheliomas ( Fig. 6.11A to C ). Other subtypes of mesothelioma include sarcomatoid mesothelioma ( Fig. 6.12 ) and mixed-type (containing components of both epithelioid and sarcomatoid) mesothelioma ( Figs. 6.13 , 6.14 ). The main reason to determine the histologic subtype of mesothelioma is that patients with sarcomatoid mesothelioma often have a much worse prognosis than those with epithelial mesothelioma. The microscopic pathologic diagnosis of malignant mesothelioma can sometimes be more challenging, because the disease can be confused pathologically with adenocarcinoma of the lung. However, several tumor markers can help distinguish adenocarcinoma from malignant mesothelioma ( Table 6.1 ). Mesotheliomas express the Wilms’ tumor protein (WT1) and calretinin, which are not expressed by lung adenocarcinomas ( ).Unlike adenocarcinomas, mesotheliomas do not express thyroid transcription factor 1 (TTF1) or carcinoembryonic antigen (CEA). These immunohistochemical features help distinguish mesothelioma from adenocarcinoma ( Fig. 6.15 ). In addition, electron microscopy has been used in the diagnosis of mesothelioma ( Fig. 6.16 ). Furthermore, two serum markers, osteopontin and soluble mesothelin-related (SMR) proteins, have also recently emerged as potential diagnostic markers in mesothelioma and are being evaluated in several prospective series ( ). These serum markers may help in screening individuals who were exposed to asbestos and thus at risk for developing mesothelioma before clinical and/or radiographic evidence of mesothelioma.