Abstract
This chapter describes the evolution of therapy for locally advanced breast cancer. Optimal management requires coordinated multidisciplinary care. Current treatment paradigms for LABC include use of neoadjuvant chemotherapy; resection, usually with mastectomy and axillary lymph node dissection; and adjuvant radiation to the chest wall or breast and regional nodes.
Keywords
locally advanced breast carcinoma, multimodality therapy, locoregional recurrence, postmastectomy radiation therapy, neoadjuvant chemotherapy
Patients presenting with locally advanced breast cancer (LABC) are at significant risk for locoregional recurrence and distant metastatic disease. Optimal treatment paradigms have changed over time and now include multimodality therapy with systemic therapy, resection, and radiation. Sequence of treatments depends on extent of disease at presentation and degree of resectability at presentation. The role of neoadjuvant chemotherapy is expanding, particularly in patients with triple-negative, HER2-positive, clinically node-positive disease, and in those with unresectable disease. Combination chemotherapeutic regimens are used and most often delivered in a dose-dense fashion. Most often, modified radiation mastectomy is used for surgical management of LABC. Some select noninflammatory breast cancer (IBC) patients with good response to neoadjuvant chemotherapy (NAC) and limited disease extent may be considered for BCS. The role of axillary staging other than axillary lymph node dissection using sentinel node biopsy, especially after NAC, is evolving and under investigation. Locoregional radiotherapy targets the areas at risk: the chest (or breast) and regional nodes. Optimal outcomes require multidisciplinary coordination and delivery of care. Increasingly, treatments will be response adapted and tailored for each patient based on individual risk for recurrence and tumor biology.
This chapter describes the evolution of therapy for LABC. Optimal management requires coordinated multidisciplinary care. Current treatment paradigms for LABC include use of NAC, resection, usually with mastectomy and axillary lymph node dissection, and adjuvant radiation to the chest wall or breast and regional nodes.
Definition
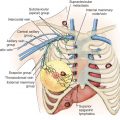
An exact definition of LABC varies among sources and as the stage groupings and TNM definitions have changed in more recent editions of the American Joint Committee on Cancer (AJCC) staging manual. Generally, patients with LABC have stage III disease at presentation with large primary breast cancers (cT3, >5 cm) and involved regional nodes; breast cancers with involvement of the skin and/or chest wall, satellite nodules, breast edema, or a combination thereof (cT4a, cT4b, and cT4c), clinical presentation of IBC (cT4d), or advanced regional nodal disease with either bulky, fixed, matted nodes, presence of infraclavicular, supraclavicular, or internal mammary nodal disease (cN2 and cN3). IBC is a clinical diagnosis on the basis of erythema and/or edema involving a third or more of the skin of the breast with rapid onset ( Fig. 53.1 ).
Some have included stage IIB large node-negative breast cancers (cT3N0) in this grouping. Others have used descriptors such as “fixed” or “skin involvement,” but these do not always coincide with staging definitions of skin ulceration or chest wall invasion. Others have included patients spanning IIB to III disease, including node positivity in general because treatment approaches are similar, particularly with expanded use of neoadjuvant therapy.
Stage grouping for LABC has changed over time. Under the seventh edition AJCC, LABC patients would be stage III patients. In previous staging editions, LABC would include both stage III and select stage IV disease (supraclavicular nodal involvement without distant metastases). Supraclavicular nodes were recategorized as N3 disease and no longer M1, and infraclavicular nodes were added to the nodal stage groupings. Additionally, the clinical definition of inflammatory carcinoma is reiterated.
Within LABC, there is significant variability in extent of disease, treatment pathways, and outcomes. Historically and practically, LABC has been divided into operable and inoperable disease. This is reflected in treatment algorithms within the National Comprehensive Cancer Network (NCCN) Breast Cancer Guidelines. Although in the NCCN Guidelines, the term “LABC” is specified on the inoperable pathway, stage III breast cancers are included on pathways for either upfront resection or neoadjuvant chemotherapy followed by resection.
Incidence
Incidence of LABC varies based on the definition used. In 2015 to 2016, there were approximately 250,000 new cases of breast cancer diagnosed and 41,000 deaths in the United States. Over the past 30 years, mortality due to breast cancer has declined significantly (36% from 1989 to 2012). This is primarily a result of early detection due to mammographic screening and education. Fortunately, the rates of LABC have similarly declined. LABC remains a global problem, however, with patients frequently presenting with advanced disease.
In 2011, there were approximately 16,000 T3 breast cancers and 70,000 node-positive cancers. Inflammatory carcinomas account for 4000 cases annually. A Surveillance, Epidemiology, and End Results (SEER) report on incidence of LABC and IBC between 1988 and 2000 showed an increased incidence rate (per 100,000 woman years) of IBC from 2 to 2.5, whereas LABC incidence rate decreased from 2.5 to 2.
Outcomes
Historically, patients with LABC have had poor outcomes; 5-year survival rates were 25% to 45% with use of local therapy only (mastectomy, radiation, or both). Per National Cancer Database (NCDB) data from 2001 to 2002, 5-year survival was 66.7% for stage IIIA, 41% for IIIB, and 49.3% for IIIC disease. For those with regional disease at diagnosis in the United States between 2005 and 2011, 5-year relative survival rates were approximately 85% with use of systemic therapy, resection, and radiation. Using the SEER registry, median survival of IBC was 2.9 years versus 6.4 years for other LABC. From a prospective clinical trial of multimodality therapy, 15-year survival was 50% for stage IIIA patients, 23% for stage IIIB patients, and 20% for IBC, demonstrating that there are long-term survivors of LABC and IBC.
General Treatment Paradigms
Treatment paradigms for LABC generally include trimodality therapy. Multidisciplinary coordinated care is integral for optimizing outcomes in these aggressive cancers as patients are at high risk for local, regional, and distant recurrence. Early involvement of all care providers is crucial and should be maintained throughout the treatment course.
In the past, operable LABC was primarily treated with modified radical mastectomy followed by chemotherapy and postmastectomy radiation. Treatment options have evolved and now increasingly include the use of NAC followed by surgery (mastectomy and breast conserving surgery [BCS] in selected cases), with nodal assessment (axillary dissection with ongoing investigation of sentinel node biopsy) and adjuvant radiation to the chest wall/breast and regional lymphatics.
For patients with inoperable disease at presentation, treatment involves NAC followed by surgery if rendered resectable and adjuvant radiation. For those patients with persistent unresectable disease after chemotherapy, considerations include modification of systemic therapy and/or use of radiation for locoregional control with reassessment for resectability.
Criteria for inoperability were first described by Drs. Haagensen and Stout and included extensive skin edema, chest wall fixation, skin satellite nodules, parasternal deposits, fixed axillary nodes, and arm lymphedema at presentation, but not nipple retraction or skin dimpling itself. In the presence of these cardinal signs, use of radical mastectomy did not lead to permanent cure, and locoregional recurrence (LRR) exceeded 50% at 5 years. These observations led to a transition from radical surgery first to radiation as primary local treatment, then inclusion of systemic therapy, and now combined modality treatment.
Rationale for Use of Systemic Therapy
The rationale for use of systemic therapy includes improvement in overall survival and reduction in recurrence. The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis of systemic therapy trials revealed a significant improvement in survival with use of polychemotherapy and endocrine therapy as well as the influence of age on outcome. The meta-analysis is limited by absence of taxanes or HER2 directed therapies. Anthracycline-based polychemotherapy reduced the annual breast cancer death rate by 38% for those under age 50; 20% for women aged 50 to 69; and for those who were estrogen receptor (ER) positive, 5 years of tamoxifen reduced the annual breast cancer death rate by 31%.
Rationale for use of NAC had traditionally been to render unresectable disease operable. Studies investigating NAC have shown improvement in downstaging, resectability, and conversion from mastectomy to opportunity for breast conservation, without improvement in overall survival compared with adjuvant delivery, however.
A recent meta-analysis of NAC for LABC demonstrated benefit to dose-dense administration over standard schedule dosing for anthracyclines with or without taxanes with increased pathologic complete response (pCR) and overall response (OR) rates (13.5% vs. 9.2% for pCR and 52.5% vs. 45.3% OR). The meta-analysis is limited by number of studies included (six studies), lack of data on ER or HER2 receptor status, and absence of HER2-directed therapies.
In vivo assessment of response to NAC permits potential crossover to other therapies, including change in systemic therapy, proceeding to surgery directly if resectable, and use of radiation therapy (RT). Use of response has increasingly been incorporated into current clinical trial design and correlative studies.
At present, use of NAC has expanded to include patients with lesser disease burden but in whom systemic therapy is indicated and thus given preoperatively versus adjuvantly. This is a particularly common approach in those with select intrinsic subtypes, triple-negative and HER2-positive cancers, and in those with node-positive disease.
Rationale for Postmastectomy Radiation
Original postmastectomy radiation therapy (PMRT) trials demonstrated significant improvements in locoregional control (LRC) and overall survival (OS) with use of PMRT in women with large, node-positive, or otherwise high-risk breast cancers. In the Danish 82b of premenopausal women treated with cyclophosphamide, methotrexate, and 5-flurouracil (CMF), LRR was 9% with PMRT versus 32% without ( p < .001), with improvement in 10-year OS (54% with PMRT vs. 45% in those with CMF alone; p < .001). For postmenopausal women treated with tamoxifen on the 82c trial, LRR was 8% with PMRT versus 35% without ( p < .001), also with improvement in 10-year OS (45% with PMRT vs. 36% in those with tamoxifen alone; p = .03). For premenopausal women treated with CMF on the British Columbia trial, use of PMRT was associated with improved LRC (10% vs. 26%; p = .002) and 20-year OS (47% with PMRT vs. 37% no PMRT; p = .03).
The role for PMRT is covered in Chapter 49 .
Meta-analyses investigating the role of PMRT have been conducted to address the role of locoregional radiation in improving outcomes for breast cancer. Whelan and associates evaluated 18 trials and 6300 patients with node-positive disease treated with modified radical mastectomy and systemic therapies. Patients included had stage II or III disease. The use of PMRT reduced any recurrence, odds ratio 0.69 (95% confidence interval [CI] 0.58–0.83); local recurrence odds ratio 0.25 (95% CI 0.19–0.34); and mortality, odds ratio 0.83 (95% CI 0.74–0.94).
The EBCTCG recently published an updated meta-analysis of the effects of PMRT on 10-year recurrence and 20-year survival. The 22 trials included in the analysis were conducted between 1964 and 1986 and included 8135 women. In node-negative women, no effect of RT was seen. In 1314 women with 1 to 3 nodes positive, RT reduced LRR (2 p < .00001), overall recurrence (relative risk [RR] 0.68, 95% CI 0.57–0.80, 2 p = .00006), and breast cancer mortality (RR 0.80 95% CI 0.67–0.95, 2 p = .01). The same effect was seen in the subgroup of 1133 women treated with systemic therapy. For the 1772 women with four or more lymph nodes involved, RT had similar effects with improvement in LRR (2 p < .00001), overall recurrence (RR 0.79, 95% CI 0.69–0.90, 2 p = .0003), and reduction in breast cancer mortality (RR 0.87, 95% CI 0.77–0.99, 2 p = .04).
Some have questioned the applicability of the results from these studies to current patients who receive systemic therapies known to be more effective, prolonged endocrine therapy, often have earlier detection, have a greater number of lymph nodes routinely evaluated with axillary lymph node dissections, and receive modern RT techniques. However, at this time, radiation after mastectomy is recommended for patients who present with LABC.
Locoregional Recurrence Rates Without PMRT
Studies of mastectomy with systemic therapy and no PMRT can be used to evaluate LRR risks and patterns of failure in the absence of RT. In the Danish 82b/c trials with 18 years of follow-up, LRR was 49% with the chest wall being the most common first site of recurrence. In the British Columbia trial, 20-year LRR was 21% for women with one to three nodes positive and 41% for four or more involved nodes. Data from the MD Anderson Cancer Center reports and the Eastern Cooperative Oncology Group (ECOG), National Surgical Adjuvant Breast and Bowel Project (NSABP), and International Breast Cancer Study Group trials have been published that include 10-year rates of LRR without PMRT ( Table 53.1 ).
| Trial | No. of Patients | LOCOREGIONAL RECURRENCE RISK (%) | Follow-Up (y) | |
|---|---|---|---|---|
| 1–3 LNs+ | ≥4 LNs+ | |||
| MDACC (Katz et al. ) | 1–3 LNs+ 466 ≥4 LNs+ 419 | 10 | 4–9 LNs 21 ≥10 LNs 22 | 10 |
| ECOG (Recht et al. ) | 1–3 LNs+ 1018 ≥4 LNs+ 998 | 13 | 29 | 10 |
| NSABP (Taghian et al. ) | 1–3 LNs+ 2957 ≥4 LNs+ 2784 | 13 | 4–9 LNs 24 ≥10 LNs 32 | 10 |
| IBCSG (Wallgren et al. ) | 1–3 LNs+ 2404 ≥4 LNs+ 1673 | 13–25 | 4–9 LNs 26–35 ≥10 LNs 26–48 | 10 |
Patterns of recurrence in these series were similar to those from the PMRT trials with local recurrence at the chest wall being most common, followed by the supraclavicular fossa, axillary apex, or infraclavicular region. Failure in the dissected axilla is uncommon, and internal mammary nodal recurrence is rare.
Guideline Statements for PMRT and LABC
Given the results of the PMRT trials and risks for LRR without RT in high-risk patients, an American Society of Clinical Oncology consensus guideline recommended PMRT for patients with four or more nodes positive and those with T3 or stage III disease. For patients presenting with LABC, PMRT was recommended. At that time, the authors acknowledged that there was limited randomized data for T3 and operable stage III LABC and for PMRT in the setting of NAC.
Cancer Care Ontario guideline recommendations on locoregional therapy of LABC attempt to address current treatment questions. In general, mastectomy is recommended as the standard of care for patients with LABC, with BCS to be used on a selective basis and not for patients with inflammatory carcinoma. PMRT is recommended for patients with LABC and includes treatment of the chest wall or breast and regional nodal basins. After NAC, adjuvant radiation is recommended for LABC patients, even those with complete pathologic response. Axillary dissection is recommended for nodal staging in LABC because data regarding use of sentinel lymph node biopsy in this setting are limited. Options provided for subsequent management in the setting of progression or lack of response to initial NAC include crossover to a new systemic therapy regimen, immediate surgery if feasible, or radiation with multidisciplinary input and care coordination.
The American College of Radiology convened an expert panel to review the literature for treatment of LABC and updated their Appropriateness Criteria in 2016. The authors highlight the importance of coordinated multimodality care using chemotherapy, surgery, and radiation for optimal outcomes and appropriate initial staging imaging to define extent of disease and subsequent response to NAC. They too acknowledge that few randomized trials specifically address the role of radiation in this setting but recommend RT after mastectomy for most patients, although optimal targets, volumes, and techniques have not been defined. They additionally note that breast conservation may be employed selectively in noninflammatory LABC patients with good response to NAC. Data on breast reconstruction options and RT related toxicities are presented as well.
Studies for LABC
There are limited studies specifically addressing LABC. The available data for radiation in the setting of LABC and with use of NAC are reviewed. Original studies of LABC mostly included patients with operable disease and included use of one modality, then progressed to combinations of treatment modalities and sequence. Subsequent or current trials include few or any LABC patients; in many trials, LABC is an exclusion criterion.
RT Alone for Operable LABC
Recognizing the significant risks for recurrence and death in patients with LABC treated with mastectomy alone, an alternative approach was to use radical radiation in this setting. Use of high-dose irradiation alone in LABC patients treated from 1960 to 1972 demonstrated potential for local control rates greater than 70% but was associated with increased late complications, including fibrosis. In a series of 137 patients with nonmetastatic LABC treated with radiation alone, 90% initial clinical response, 5-year local control of 54%, distant DFS of 28%, and overall survival of 30% were seen. Node-negative disease, use of excisional biopsy, dose of greater than 60 Gy, and use of systemic therapy were associated with improved local control. A follow-up publication of 192 patients with T3–4 or N2–3 disease (some of whom had excisional biopsy and with greater use of systemic therapy) demonstrated improvement in survival (41% at 5 years and 23% at 10 years). Crude local control was nearly 80% and influenced by dose of greater than 60 Gy delivered.
Neoadjuvant Chemotherapy Followed by Either Resection or Radiation
The Cancer and Leukemia Group B (CALGB) investigated the addition of NAC to primary local therapy, either resection or radiation. This original trial of upfront systemic therapy in stage III breast cancers included patients with cT3N1–2 or cT4N0–2 LABC. Patients were treated with chemotherapy for three cycles (cyclophosphamide, doxorubicin, 5-fluoruracil, vincristine, and prednisone), then reassessed for operability and randomized to either mastectomy or radiation, followed by adjuvant chemotherapy. There were 87 patients evaluable for resection versus radiation with no significant differences noted by local therapy (approximately 50% of patients relapsed, half were local), and there was no survival difference. Radiation was delivered to the breast and regional nodes (supraclavicular, axillary, and internal mammary nodes) to 50 Gy with boost to residual disease (22 Gy to breast and 15–20 Gy to axillary nodes). The study did demonstrate improvement in disease control with systemic therapy in addition to local therapy alone. The median disease control was 2 to 2.5 years. Pre- or perimenopausal status or those presenting with inflammatory disease had worse outcomes.
Resection Followed by Systemic Therapy and Either Observation or Radiation
The addition of RT to resection and systemic therapy was evaluated by the ECOG trialists. Inclusion criteria were those with operable noninflammatory LABC: T4 disease, T3N1–2 or muscle involvement, or T1–2 disease fixed to muscle or with N2 disease. Patients had upfront mastectomy followed by six cycles of systemic therapy with cyclophosphamide, doxorubicin, 5-fluorouracil, tamoxifen, and fluoxymesterone. Those without distant disease were randomized to either observation or radiation. Radiation was delivered to the chest wall and regional nodes to a total dose of 46 to 50 Gy. Patterns of failure differed by treatment regimen. Use of RT was associated with a reduction in LRR (24%–15%) with increased rate of distant relapses (50% vs. 35%), although no difference in time to relapse or overall survival was seen. Patients who developed recurrence after observation were treated with radiation for local control.
Neoadjuvant Systemic Therapy and Radiation for LABC
The French evaluated the potential for upfront systemic therapy and radiation in noninflammatory LABC patients. On this trial, 120 patients received four cycles of anthracycline-based chemotherapy followed by preoperative radiation consisting of 45 Gy to the breast and nodal regions. Patients then received additional local therapy (resection or radiation) and further chemotherapy. The option for subsequent local therapy was response stratified. Those with larger amounts of residual disease proceeded to modified radical mastectomy (MRM), whereas those with less than 3-cm residual disease underwent wide local excision and axillary lymph node dissection, and those with clinical complete response received a radiation boost without resection. The 10-year local failure rates were 4% with MRM, 23% with wide local excision + RT, and 13% with RT alone. Factors associated with DFS included clinical stage, nodal stage, and tumor response. This trial and preceding data from use of radiation alone defined a role for potential preoperative RT in downstaging and local control in LABC unresponsive to NAC.
Inoperable LABC
Patients with inoperable LABC at presentation and resistance to chemotherapy are a rare subset of patients who present a significant treatment challenge. A total of 38 such patients were identified from five institutional trials of NAC (4.4% of patients enrolled). These patients had T3, stage III, or stage IV (isolated supraclavicular involvement and no distant metastatic disease), remained inoperable after upfront anthracycline-based therapy, and then received radiation to the breast and lymph nodes (median dose 45–50 Gy with 10-Gy boost). After RT, 84% were able to proceed to mastectomy, and those treated with all three modalities had improved local control and DFS. Although limited in numbers, the response rates to NAC and RT were informative: 18% clinical response to NAC and additional 26% with RT, and nodal response of 23% with NAC and additional 58% with RT. As in other similar trials of LABC, advanced nodal stage was associated with worse outcomes than primary disease extent. In this cohort, patients expected to have extremely poor outcomes, but OS was 46% at 5 years and 20% at 10 years; distant disease–free survival was 32% at 5 years and 19% at 10 years, demonstrating for the importance of intensive multimodality therapy in the setting of poor upfront response.
Inflammatory Breast Carcinoma
IBC is a subset of LABC associated with high rates of LRR, distant metastases, and poor survival outcomes. Historically, patients treated with radical resection developed rapid occurrence of chest wall disease and radical radiation resulted in limited long-term local control and poor survival. The addition of systemic therapy to local treatments improved outcomes. A review of IBC patients treated with doxorubicin-based systemic therapy and radiation from 1974 to 1993 showed response to neoadjuvant therapy influenced risk for local recurrence and those who also received mastectomy had lower rates of local failure.
Current treatment for IBC includes NAC, MRM, and PMRT. A retrospective review of 256 IBC patients treated at a single institution between 1977 to 2004 showed that patients who received all three treatment modalities had improved outcomes versus those who did not (5-year LRC 84% vs. 51%, distant metastasis–free survival 47% vs. 20%, and OS 51% vs. 25%, p < .0001). Factors associated with LRC included response to chemotherapy, surgical margin status, number of lymph nodes involved, and use of taxanes. In patients with limited response to systemic therapy, nonnegative margins, and young age, dose escalation from 60 to 66 Gy given twice-daily improved local control. PMRT fractionation and dose evolved over time, shifting from once daily to twice daily treatments, and increase in total dose. This accelerated hyperfractionated course of PMRT with dose escalation to 66 Gy was associated with improvement in LRC, DFS, and OS and was delivered as 51 Gy to the chest wall and regional nodes with a 15-Gy chest wall boost in 1.5-Gy fractions given twice-daily. Others have reported local control rates of 87% with once daily treatment and use of skin bolus after taxane-based NAC and mastectomy. There is not a single optimal locoregional radiation treatment approach for IBC, although use of acceleration, bolus, and/or dose escalation should be considered, particularly in patients with poor response to NAC.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree