16 Radiation Therapy for Colorectal Adenocarcinoma: External Beam and Intraoperative Radiation Therapy
Background
In 2009, an estimated 40,870 new cases of rectal cancer and 106,100 cases of colon cancer were diagnosed in the United States.1 Despite aggressive surgical management, 5% to 40% of rectal cancer patients and 30% of advanced colon cancer (T4) patients will experience locoregional relapse.2–4 Radiation has been shown to decrease the risk of local recurrence after blunt and mesorectal excisions.5,6 The German rectal cancer trial demonstrated that preoperative chemoradiation (CRT) improves local control and sphincter preservation and results in less treatment-related toxicity compared with postoperative CRT.7 Relapse rates for rectal cancer are especially high when tumors are adherent to or invading adjacent structures (T4). In these cases, even aggressive management with neoadjuvant CRT and total mesorectal excision (TME) can often result in margin-positive resections. In turn, a positive surgical resection margin is associated with an increased risk of local recurrence. Without treatment, patients with pelvic recurrences may experience severe pain and in general have a median survival of about 8 months.8 Palliative surgery alone improves survival up to a median of 11 months.2 Although radiation therapy or chemotherapy plus radiation can successfully palliate pain, 5-year survival rates for either therapy when used alone are less than 5%.9,10 When colorectal cancers recur, a multimodality approach is required.
After decades of treating locally advanced colorectal cancer (CRC) with 5-fluorouracil (5-FU) and radiation, newer cytotoxic and biologic agents are now available. Combinations of cytotoxic agents (e.g., irinotecan and oxaliplatin) and targeted therapies (e.g., cetuximab and bevacizumab) have led to increases in disease-free and overall survival in patients with colorectal cancer.11–15 With the advances in adjuvant systemic treatment, patients with colorectal cancer are now living longer. As such, durable control of the primary tumor site has renewed importance. In addition to appropriate aggressive surgical management (e.g., mesorectal excision, en bloc resection of involved organs, and so on), there has been a renewed interest in intraoperative radiation therapy (IORT) for locally advanced or locally recurrent pelvic adenocarcinoma. Specifically, some clinicians have advocated that preoperative CRT followed by IORT and adjuvant chemotherapy may lead to improved outcomes for patients.
External Beam Radiation Therapy for Rectal Cancer
Sauer and colleagues7 conducted a randomized clinical trial that established preoperative CRT and surgery followed by maintenance chemotherapy as the standard of care for clinical stage T3, T4, or node-positive rectal cancer. Early studies evaluating preoperative therapy used hypofractionated radiation alone. Specifically, the Swedish rectal trial demonstrated a statistically significant improvement in local control and survival with the addition of radiation alone (25 Gy in five fractions) to the pelvis before a non–TME.6 This trial showed that local therapy alone can improve survival after surgical resection. The Dutch rectal trial evaluated whether radiation therapy (25 Gy) was still necessary after a TME.16 Although local control was improved with TME, there remained a statistically significant improvement in local control with the addition of radiation, but no improvement was seen in overall survival.
In the United States, conventionally fractionated preoperative irradiation alone is supported by several single-institutional studies.17 Three more recent randomized clinical trials from Europe have investigated the role of preoperative irradiation with or without chemotherapy. Two of these studies (EORTC 22921 and FFCD 9203) demonstrated a significant improvement in local control when 5-FU-based chemotherapy was added to radiation (45 Gy) in the neoadjuvant setting.5,18 As expected, acute toxicity was increased with the addition of chemotherapy, as had been noted in the French trial (FFCD 9203).5 A third trial from Poland compared a short-course (25 Gy) with a standard-course radiation therapy regimen (45 Gy). No statistically significant difference was found with regard to local control, survival, or late toxicity between the two arms, although the trial had several flaws including inconsistencies with maintenance chemotherapy after surgery.19
Although local control has been improved with neoadjuvant CRT, many patients still succumb to metastatic disease. Therefore, several studies have explored adding a second chemotherapeutic agent concurrent with 5-FU. Oxaliplatin has been shown to be efficacious in the metastatic setting and now appears to be reasonably well tolerated when added to 5-FU and radiation therapy.14,20,21 Targeted therapies such as erlotinib (epidermal growth factor tyrosine kinase inhibitor) and bevacizumab have also been evaluated in the neoadjuvant setting and may result in further improvements in local control and survival.14,15
Radiation Therapy Treatment Planning
Generally, patients are treated prone on a belly board with three fields of radiation (one posterior and two laterals). Patients undergo computed tomography simulation, and the primary tumor (tumor bed if adjuvant) and iliac vessels (representing lymph node areas at risk) are delineated. Standard borders include L4/L5 vertebral bodies superiorly and the bottom of the ischial tuberosity inferiorly. Lateral borders correspond to the lateral aspect of the iliac lymph nodes or approximately 1.5 cm lateral to the pelvic brim. To limit the dose to normal structures, many centers have begun using intensity-modulated radiation therapy (IMRT) for localized rectal cancer in the neoadjuvant setting.22
Adjuvant Radiation Therapy With or Without Chemotherapy
Several classic trials have examined the use of adjuvant radiation alone or in combination with chemotherapy. Several studies demonstrated that chemotherapy and radiation improved local control and survival.23–25 The method of administration of chemotherapy appears to be important for obtaining optimal results. Infusional 5-FU was found to be superior to bolus 5-FU. The toxicity between the regimens also differed, with infusional 5-FU causing more diarrhea and bolus 5-FU resulting in pancytopenias.26–28 The timing of chemotherapy may also be important according to a recent randomized study. Starting radiation therapy with the first cycle of chemotherapy led to a better outcome compared with the traditional “sandwich” approach (chemotherapy alone, combined CRT, chemotherapy alone), which was used in most trials.29 Thus, infusional 5-FU with radiation therapy can be considered a standard adjuvant therapy, and it may be reasonable to begin radiation with the first cycle of chemotherapy. Capecitabine, an orally active prodrug of 5-FU has been shown to be equally efficacious in the adjuvant and neoadjuvant setting.30
Intraoperative Radiation Therapy: Overview
Intraoperative radiation therapy (IORT) refers to the delivery of high doses of radiation to the tumor bed at the time of surgical resection. IORT has been advocated as an adjunct to external beam radiation therapy (EBRT) to escalate the dose of radiation at the site of resection. IORT can be delivered using electrons (intraoperative electron beam radiation therapy [IOERT]) or with brachytherapy catheters.31,32 Both methods involve retraction of mobile normal structures, most notably bowel. Structures that cannot be retracted out of the treatment field (e.g., blood vessels, ureters, and peripheral nerves) are shielded using lead.
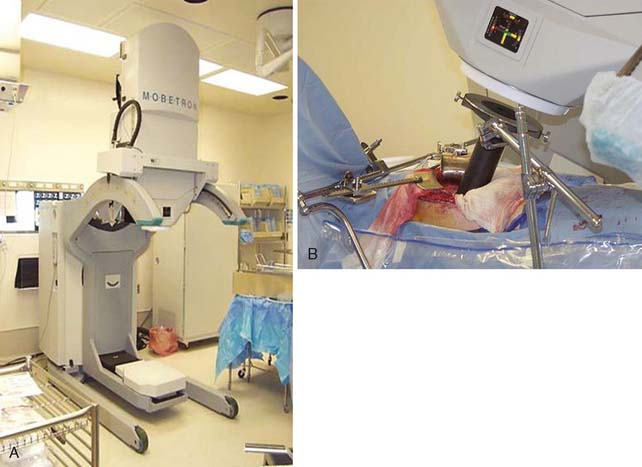
IOERT is administered using a standard or mobile linear accelerator (Mobetron, IntraOp Medical Corporation, Sunnyvale, California; Linac Systems, LLC, Albuquerque, New Mexico), which can deliver various electron beam energies (i.e., doses) to prespecified tissue depths33 (Fig. 16-1). With IOERT, various cone shapes and sizes can be used to adapt to diverse tumor bed volumes. One limitation of IOERT is that it may require several overlapping IOERT cones to adequately cover the area of interest. Overlapping cones may result in radiation “hot spots” that may increase toxicity, especially peripheral neuropathy.34 To decrease these hot spots, some institutions (e.g., Mayo Clinic, Rochester, Minnesota) use rectangular or ellipsoid applicators while eliminating the need for overlapping circular fields. In contrast, the University of Heidelberg Department of Radiation Oncology uses horseshoe-shaped applicators that simplify abutting two smaller fields.35 For the Mobetron, square and rectangular applicators are being developed that allow for field sizes of up to 8 cm × 20 cm as a single field size (personal communication, Mobetron).
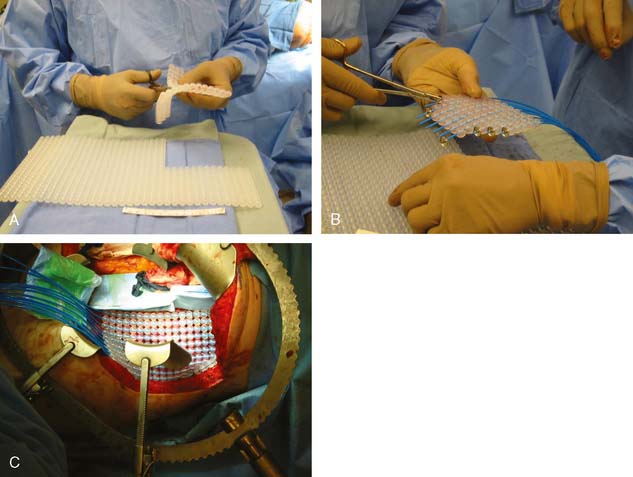
Brachytherapy involves placement of catheters in the tumor bed after en bloc resection of the tumor. The catheters are placed individually or into a silicone flap (Freiburg Flap, Nucletron, Veenendaal, The Netherlands, or Harrison-Anderson-Mick [HAM] applicator, Mick-Radio Nuclear Instruments, Inc., Mount Vernon, New York), which can be cut to conform to the tumor bed (Fig. 16-2A–C). IORT brachytherapy can be administered using either low-dose rate IORT (LDR-IORT) or high-dose rate IORT (HDR-IORT).
In contrast, with HDR-IORT the complete dose of IORT is administered at the time of surgery. Specifically, HDR-IORT is administered through a wire with a single iridium source (Ir-192) at the end, which passes through catheters embedded in a silicon flap at evenly spaced 1-cm intervals. A robot guides the brachytherapy wire through the catheters and stops at “dwell” positions that have been designated by the radiation treatment plan. These dwell positions and times can be used to manipulate the dose at various points along the flap. With HDR-IORT, the tumor bed can be covered by a single flap, in contrast to IOERT, which may require several overlapping IOERT cones. In addition, HDR-IORT results in a higher surface dose (HDR 200% of prescribed dose versus IOERT 75%–95% of prescribed dose) with a lower dose at 2 cm (HDR 30% versus IOERT 70%–100%) compared with IOERT.33 Unlike LDR-IORT, with HDR-IORT the flap and catheters are removed immediately after treatment has been delivered, thereby limiting radiation exposure to physicians and surgical staff. However, compared with IOERT, HDR/LDR brachytherapy treatment and procedure times are typically longer and depend on the source strength at the time of the procedure (Table 16-1).
Table 16-1 Potential Differences Between IOERT and HDR-IORT
| IOERT | HDR-IORT | |
|---|---|---|
| Treatment time | 2–4 minutes | 5–30 minutes |
| Procedure time | 30–45 minutes | 45–120 minutes |
| Treatment sites | Accessible locations | Depth at risk ≤0.5–1.0 cm from surface |
| Surface dose | Lower (75–93%) | Higher (200%) |
| Dosimetric homogeneity | ≤10% variation | ≥100 variation |
| Dose at depth (2 cm) | Higher (70–100%) | Lower (30%) |
HDR-IORT, high-dose rate intraoperative radiation therapy; IOERT, intraoperative electron beam radiation therapy.
In general, the biologic effectiveness of single-dose IORT is thought to be equivalent to 1.5 to 2.5 times the same total dose of fractionated EBRT.36 Therefore, adding 15 Gy of IORT to 45 Gy of EBRT is equivalent to an EBRT dose of 75 to 87.5 Gy—the dose range believed to be most effective at controlling microscopic residual disease.
Patient Selection for Intraoperative Radiation Therapy
All patients being considered for pelvic exenteration should undergo preoperative computed tomography (CT) or magnetic resonance imaging (MRI) to evaluate the extent of local disease and to exclude extrapelvic metastasis. Fluorodeoxyglucose-positron emission tomography (FDG-PET) can be used as an adjunct to CT to better identify metastatic disease. The accuracy of CT in the preoperative assessment of rectal cancer has been reported to range from 55% to 72%.37,38 MRI has been reported to be superior to CT in accurately predicting tumor invasion through the bowel wall. In addition, MRI has been reported to be more accurate than CT in the identification of sacral bone and piriform muscle involvement.39 However, even with MRI it can be difficult to reliably distinguish radiation changes from tumor involvement in patients with pelvic sidewall abnormalities.40
Most studies indicate that 24% to 64% of patients with locally recurrent rectal cancer can have resection with negative margins.41–48 In patients with locally advanced disease, extirpation of structures such as the bladder, reproductive organs (e.g., vagina, uterus), or bony structures including the sacrum-coccyx may be necessary. Although sphincter preservation is a goal of resection, oncologic principles and adequate margins should not be compromised to maintain sphincter function. In patients with metastatic disease who have synchronous locally advanced or locally recurrent disease, IORT should be considered in conjunction with local extirpation of the pelvic tumor—usually after a course of chemotherapy to ensure that the systemic burden of disease is stable or responsive.49
Review of Literature on IORT
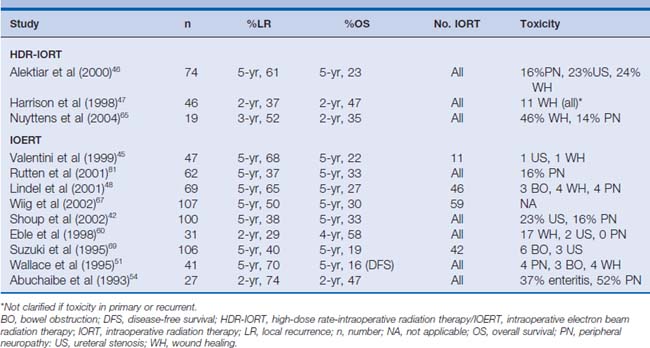
Early studies investigating advanced and recurrent rectal cancer have demonstrated that local control and survival are stage- and margin-dependent.43,50–54 For locally advanced disease, the 5-year local recurrence and survival rates range between 5% to 37% and 46% to 70%, respectively. These studies also show that the rate of local control and survival for primary locally advanced rectal cancer is clearly superior to that for recurrent disease (recurrent locoregional disease: local recurrence 21%–74%, overall survival 16%–41%) (Tables 16-2 and 16-3).
Table 16-2 Studies Evaluating HDR-IORT or IOERT in Patients with Locally Advanced/Unresectable Primary Rectal Cancer
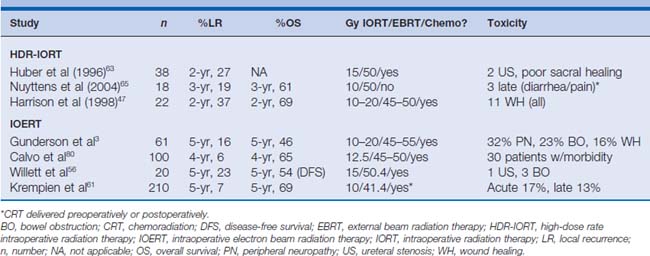
The Radiation Therapy Oncology Group (RTOG) reported a phase I/II study that evaluated patients with advanced, unresectable, or recurrent carcinoma of the rectum.55 Of the 86 evaluable patients, 42 patients received either IORT alone (n = 15) or in combination with EBRT (n = 27). The investigators reported that local control rates were dependent on the amount of residual disease after surgical resection before IOERT. Specifically, patients who had grossly negative surgical margins (e.g., either R0 or R1 resection) had a significantly better 2-year actuarial local control rate and overall survival rate compared with patients who had gross residual disease (e.g., R2 resection) at the IOERT site (local control 88% versus 77%, P = .001; overall survival 48% versus 10%, P = .006; R0/R1 versus R2, respectively). These findings have been corroborated by Willett and colleagues,56 who similarly reported a 5-year disease-free survival rate of only 6% for patients with locally advanced rectal cancer who underwent IORT after an R2 resection compared with 54% when the margins were grossly negative. Data from a recent update of this study confirm the long-term implications of margin status before IOERT therapy (5-year overall survival rate, no gross residual disease 40% versus gross residual disease 13%; P = .0001).48
To see whether IORT might improve outcomes after state-of-the-art rectal surgery alone, Pacelli and colleagues57 compared preoperative radiation therapy (no chemotherapy) plus IORT after rectal resection with TME versus TME alone for patients with middle and lower T3 rectal cancer. From 1991 to 1997, 113 patients received either preoperative radiation therapy (38 Gy) plus IORT (10 Gy; n = 69) or TME alone (n = 44). Postoperative complications were comparable in the two groups. Patients who received preoperative radiation therapy followed by TME and IOERT tended to have a longer 5-year disease-specific survival rate (81%) compared with patients who underwent TME alone (58%) (P = .052). The differences in local control, however, were more dramatic. Specifically, local recurrence at 5 years was more than three times more common in the TME-alone group (23.2%) compared with the combined-modality group (6.6%) (P = .017). The data from this study demonstrated the superiority of a combined-modality approach that includes preoperative radiation therapy, TME, and IOERT over TME alone in patients with T3 rectal cancer. This study, however, was not able to address the relative benefit of IORT itself (e.g., preoperative radiation therapy, TME, and IOERT versus preoperative radiation therapy, TME, without IOERT).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree