Raj Palraj, Bettina M. Knoll, Larry M. Baddour, Walter R. Wilson Keywords antimicrobial therapy; blood culture; culture-negative endocarditis; echocardiography; emboli; endocarditis; enterococcal infections; fungal endocarditis; HACEK infections; indications for surgery; infection; management; perivalvular complications; prophylaxis; prosthetic valve; staphylococcal infections; streptococcal infections; valve surgery
Prosthetic Valve Endocarditis
During the second half of the 20th century, prosthetic heart valve replacement surgery represented the most important advancement in the treatment of patients with severe valvular disease. Prosthetic valve replacement markedly improves patient’s functional class, hemodynamics, left ventricular function, and survival. Prosthetic valve endocarditis (PVE), a microbial infection involving the valve prosthesis or repaired native heart valve with placement of an annuloplasty ring, is a rare but potentially lethal complication of prosthetic valve surgery. Despite diagnostic and therapeutic advances, PVE is characterized by high rates of relapse, morbidity, and mortality.
With the virtual elimination of rheumatic heart disease in developed countries where prosthetic valve surgery is more generally available, degenerative valvular disease—calcific aortic stenosis—has become the most common cause for prosthetic valve replacement in industrialized countries. As a result of the increase in prosthetic valve implantations performed for degenerative valvular disease in elderly patients and longer survival of patients with prosthetic valves, the number of patients at risk of PVE will continue to increase in the developed world.
Epidemiology
The incidence of PVE ranges from 1% to 6% of prosthetic valve implantations, or 0.3% to 0.6% per patient-year.1–3 PVE accounts for 16% to 33% of all definite cases of infective endocarditis (IE), according to data from retrospective studies of single and multicenter tertiary care units in developed countries,3–10 and from prospective national,11–13 European,9 and international multicenter observational14 studies. Risk factors associated with the development of PVE include male sex, previous native valve endocarditis, and long cardiopulmonary bypass time for prosthetic valve placement.15 The cumulative risk of developing PVE is highest within the initial 12 months after replacement surgery, with a peak during the first 2 months.14,16
The prosthetic valve is vulnerable to secondary microbial seeding during the early postimplantation period when valve endothelialization has yet to occur. The mean age of patients with PVE is 65 years; the range is 50 to 74 years.8–11,14,17,18 The risk of PVE is higher in patients who undergo valve replacement surgery during active IE, especially in the setting of an unknown pathogen or insufficient antibiotic treatment.19–23
The mechanical prostheses appear to have a slightly higher risk during the first 3 months postsurgery, and the bioprosthetic valves appear to have a slightly higher risk after 12 months postsurgery,20,21,24 likely as a result of degenerative changes in the bioprosthetic leaflets. However, the cumulative risk of PVE appears similar between mechanical and bioprosthetic valves.22,24–27 The frequency of PVE also appears to be similar for both aortic and mitral prosthetic valves.
Microbiology
The microbiology of prosthetic valve endocarditis depends on (1) time of onset of PVE (early vs. late PVE) and (2) site of acquisition (community vs. health care associated).
Early- and Late-Onset Prosthetic Valve Endocarditis
PVE has been categorized as early-onset PVE and late-onset PVE on the basis of the time period between prosthetic valve replacement and the manifestation of endocarditis. The microbiology of early- and late-onset PVE is different.14,19,28,29 Early-onset PVE has been due to infections acquired intraoperatively or during the immediate postoperative period. Staphylococcus aureus, coagulase-negative staphylococci (CoNS), diphtheroids, fungi, and nosocomial aerobic gram-negative bacilli are the most common pathogens in early PVE.10,14,29–34 In the past, the time limit was arbitrarily chosen as 60 days,7,14,19 to differentiate perioperative infections from late community-acquired infections. However, less virulent organisms acquired perioperatively may manifest many months after surgery. The peak incidence of CoNS endocarditis is between 60 and 365 days after valve implantation.14 Hence, 1 year appears to be a better reference point to distinguish between early and late PVE.10,18,29,35 There has been a sharp decline in the proportion of early PVE from 60% of all PVE cases in studies published in the 1970s31,32,34 to about 10% to 20% in more recent studies10,14,30 without significantly affecting the overall rate of PVE.2,10,14,24,33 The reduced early PVE rate is the consequence of better infection prevention and control practices, appropriate use of antimicrobial prophylaxis, improvements in design of prosthetic valves, and better surgical techniques.
Late PVE is usually considered acquired in the community, unrelated to surgery or perioperative period. However, as a result of changes in the health care delivery system and the longer survival of patients with PVE, health care–associated PVE cases are increasing in the late period.10 In some regions of the world, staphylococci have surpassed streptococci as the most frequent causative organisms of late PVE. In some hospitals, enterococci have surpassed viridans group streptococci and thus are the third most common etiologic agent of late PVE.10,14,30
Health Care–Associated Prosthetic Valve Endocarditis
Health care–associated infections have become the most important risk factor for development of PVE in recent years.* Health care–associated PVE constituted about 37% of all cases in a prospective, multinational cohort. S. aureus is now the leading causative organism of PVE, with increases in frequency of methicillin-resistant strains across geographic regions.14 Major risk factors for health care–associated PVE include intravascular devices and hemodialysis. About 70% of health care–associated PVE cases are diagnosed within the first year after prosthetic valve implantation and more than 60% occur beyond 60 days postsurgery. Many aerobic gram-negative bacilli can cause health care–associated PVE, and multidrug resistance is common. Among this group, Pseudomonas, Serratia, Acinetobacter, and Stenotrophomonas spp. predominate. Fungal PVE due to Candida spp. can occur with health care exposure. Candida albicans and Candida parapsilosis are the most frequently isolated fungal pathogens of PVE. Other non-albicans Candida spp. such as Candida glabrata and Candida krusei can cause PVE.
Community-Acquired Prosthetic Valve Endocarditis
Most community-acquired PVE is caused by enterococci, viridans group streptococci, and fastidious organisms including the HACEK group (Haemophilus parainfluenzae, Aggregatibacter aphrophilus, Aggregatibacter actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella spp.).11–13,42–47
Late-onset PVE is rarely caused by Mycobacterium, Coxiella, Tropheryma, Bartonella, and Legionella spp.48–56 Aspergillus spp. have been noted in few infections and can be difficult to identify as causative pathogens because blood cultures are usually negative. Fungal stains of the resected tissue can show characteristic fungal hyphal elements. Other rare fungal pathogens include Cryptococcus neoformans and Histoplasma capsulatum.
Pathogenesis
PVE can occur due to direct microbial contamination of a prosthetic valve at the time of surgery or as a consequence of secondary hematogenous seeding from a distant infectious focus. The prosthetic valve and perivalvular tissue are vulnerable for secondary microbial seeding during the early postimplantation period when they lack protective endothelial lining.
The pathogenesis of PVE consists of several steps including (1) microbial adherence to the prosthetic valve, damaged endothelium, platelet-fibrin aggregate, and/or periprosthetic tissue; (2) recruitment and activation of monocytes, platelets, activation of extrinsic coagulation cascade resulting in an infected coagulum called a vegetation, the characteristic lesion of endocarditis; (3) persistence and growth of the microorganism within cardiac lesions leading to local tissue destruction and invasion; and (4) dislodgement of the vegetations may occur, resulting in septic emboli in distant organs such as the brain, skin, spleen, and kidneys or in the musculoskeletal system.57,58
Microbial Adherence
Microbial adherence is a critical step in the pathogenesis of endocarditis and involves complex interactions between microbial surface proteins and host extracellular matrix molecules. The endothelial lining of the heart is usually resistant to microbial infection. However, during the early postimplantation period, the prosthetic valve, cardiac annulus, periannular tissue, sewing ring, and sutures are not endothelialized. The foreign material is often coated with extracellular host matrix molecules (e.g., fibrinogen, fibrin, fibronectin, collagen, elastin, plasma proteins, platelet proteins) that can serve as ligands for microorganisms.59 Microorganisms can also adhere to and infect the sterile platelet-fibrin aggregate formed following injury or inflammation of the endothelium. The aging bioprosthesis, sutures, and sewing cuff fabric of a valve prosthesis are thrombogenic and favor deposition of fibrinogen-fibrin, fibronectin, plasma proteins, and platelets.
The microbial surface proteins that can bind to the host extracellular matrix proteins are called “microbial surface components recognizing adhesive matrix molecules” (MSCRAMMs).60 These surface components play a key role in the initiation of endovascular infections, bone and joint infections, and prosthetic-device infections. The usual pathogens of prosthetic valve endocarditis (S. aureus, CoNS, streptococci) possess abundant MSCRAMMs. CoNS possess several adhesins, including autolysin/adhesins AtlE and Aae,61,62 the fibrinogen-binding protein Fbe/SdrG,63,64 the giant 1-MDa fibrinogen-binding protein Embp, and lipase GehD.65 Additional protein and polysaccharide components like autolysin AtlE are involved in primary attachment to polymers. CoNS have emerged as important pathogens in modern clinical practice, primarily by their ability to adhere to biomaterials and form a stable biofilm.65,66,67,68 The biofilm is a slimy, slippery coat formed by a structured community of bacterial cells enclosed in a self-produced extracellular polymeric matrix adherent to solid surfaces.69,70
S. aureus has many surface proteins and nonprotein adhesins71 that play a crucial role in the pathogenesis of IE.72–76 These include clumping factors A (ClfA), fibrinogen-binding adhesins, and the bifunctional fibrinogen/fibronectin-binding protein A (FnBPA). The microbial surface proteins of streptococci that have been studied in experimental models of IE include glucans (Streptococcus sanguis, Streptococcus mutans, Streptococcus gordonii)77; Fim A (Streptococcus parasanguis)78,79; Ace, an adhesin for collagen type IV, collagen type I, and laminin; endocarditis; and Hsa, a sialic acid–binding protein (S. gordonii).80 Enterococci have biofilm-associated proteins (Enterococcus faecalis),81,82 Acm and Scm, and collagen adhesins (Enterococcus faecium).83,84
Formation and Growth of Vegetations
The microbial growth within a platelet-fibrin aggregate leads to activation of the extrinsic coagulation cascade, recruitment of monocytes, and platelets that result in the formation of vegetation. The adherent bacteria activate the extrinsic coagulation pathway by triggering release of tissue factor from monocytes that adhere to early vegetations85,86 and from endothelial cells surrounding the infected valves.87,88 They also influence recruitment and activation of monocytes.89–91 For example, the FnBPA proteins of staphylococci interact with the endothelial cells and help in recruitment of monocytes by triggering the expression of intercellular adhesion molecule (ICAM-1) and vascular cell adhesion molecule (VCAM-1), interleukin (IL)-6, IL-8, monocyte chemotactic protein (MCP-1), and tissue factor.
Microorganisms also engage and activate platelets either directly or through bridging molecules. For example, S. aureus induces platelet activation by several surface proteins. ClfA/clumping factor B (ClfB) and FnBPA/fibronectin-binding protein B (FnBPB) are the major platelet activating modulins.92–95 ClfA and FnBPA/FnBPB bind to the low-activity platelet glycoprotein IIb/IIIa (GPIIb/IIIa) receptor through fibrinogen and fibronectin bridges. The circulating specific ClfA or FnBPA/FnBPB antibodies engage the crystallizable fragment γ receptor IIA (FcγRIIA). Activation of both receptors on quiescent platelets initiates a signal transduction cascade resulting in platelet activation and aggregation. S. sanguis activates platelets by either direct interaction between the serine-rich glycoprotein A (SrpA) and the glycoprotein Ib receptor (von Willebrand factor [vWF])96 or specific antibody and complement assembly that link the surface proteins to platelet FcγRIIA and complement receptors.94,97 Strains of S. gordonii stimulate platelet aggregation directly by binding to glycoproteins Ib and IIb through the serine-rich surface glycoproteins GspB and the sialic acid–binding adhesins (Hsa).98–102
These processes result in an enhanced procoagulant and inflammatory activity in the vicinity of the infection. The thrombus serves as a focus for adherence of additional bacteria and platelets leading to growth of the vegetation.
Persistence and Growth of Microorganisms
Microorganisms are able to persist and grow within vegetations using many mechanisms to evade the host defense system. The usual pathogens of IE have reduced susceptibility to platelet-microbicidal proteins in vitro.103–106 ClfA of S. aureus inhibits phagocytosis by human polymorphonuclear leukocytes in the absence of fibrinogen.107 Fibrin-adherent streptococci are not engulfed by monocytes.86 S. aureus can internalize into intact endothelial cells through a fibronectin bridge between FnBPA and the endothelial α5β1 integrins (fibronectin receptors). This internalization of S. aureus can lead to persistent or recurrent infection by avoiding host defense and membrane-active antimicrobial agents, such as β-lactams and glycopeptides.
The biofilm over the prosthetic valve also provides a protective environment for the bacteria. The sessile bacteria within the biofilm are less susceptible to the host immune system and antibiotics than the free-floating (planktonic) bacteria. The polymeric matrix acts as a diffusion barrier to retard the diffusion of antibiotics and reactive oxidants of phagocytic cells. Bacteria within the biofilm exhibit an altered phenotype with different patterns of growth, gene expression, and protein production. Nutrient-deficient bacteria in the biofilm switch to a slow-growing metabolically quiescent persister phenotype, which is less susceptible to antimicrobial agents.108
Tissue Destruction and Invasion
Microorganisms produce various toxins and tissue-degrading enzymes that result in invasion and tissue destruction. The extent and rapidity of tissue destruction depend on the virulence of the microorganisms. S. aureus, the most common pathogen of PVE, is a virulent organism capable of inducing significant tissue destruction in a short period of time. In S. aureus, the secretion of tissue-degrading enzymes and toxins is coordinated in a growth phase–dependent manner by global regulator loci such as accessory gene regulator (Agr), the stress response regulon (SigB), and staphylococcal accessory regulator (SarA).72,73,75,76,109
Pathology
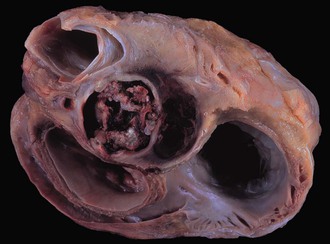
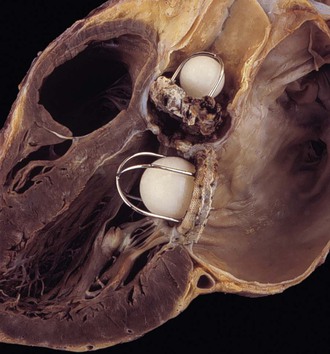
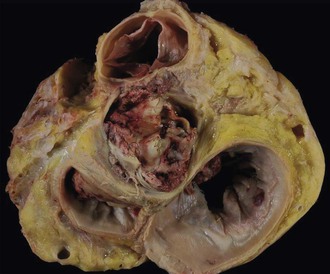
The clinicopathologic findings of PVE usually differ between mechanical and bioprosthetic valves. Mechanical heart valves are primarily made of metal or carbon alloys and are classified according to their structure as caged-ball, single-tilting disk, or bileaflet-tilting disk valves.110 Metal or carbon alloys are usually not well suited for microbial adherence. The infection of mechanical prostheses usually starts at the interface between the sewing cuff and the native tissue. The perivalvular tissue invasion can result in loosening of the sutures, causing periprosthetic leaks, or in ring abscesses (Fig. 83-1). Annular abscess or paravalvular leak was more common in mechanical PVE than bioprosthetic PVE.111 In a retrospective multicenter study at 16 tertiary referral hospitals, 148 (17%) of 872 patients with PVE had perivalvular complications of the aortic ring, including aortocavitary fistulas in 19% and nonruptured abscess in 81%.112 The ring abscess may dislodge the prosthesis from its anchorage (Fig. 83-2) and give rise to the echocardiographic appearance of a “dancing” prosthesis. Acute ventricular decompensation and congestive heart failure, due to either valve obstruction or incompetence, are important complications of PVE requiring surgery. In one study, 45% of all patients with PVE developed prosthetic dehiscence with moderate or severe valve regurgitation.112 Aortic PVE may also result in third-degree heart block if the infectious process extends to the conducting system of the heart. Ruptured ring abscesses may form fistulous tracks into cardiac chambers or into the intraventricular septum and lead to intracardiac shunting.112 The aortic PVE may result in aneurysms of the sinus of Valsalva by extending to the aortic root or aneurysms of the anterior leaflet of the mitral valve by extending through mitral-aortic fibrous continuity. The infection may also extend to involve the other valves resulting in multivalvular endocarditis (Fig. 83-3).
Bioprosthetic valves are homografts (preserved human aortic valves) or heterografts (bovine pericardial or porcine valve tissue mounted on a metal support), and the infection is usually restricted to the cusps (Fig. 83-4). The growth of thrombotic vegetations in the biosynthetic material can lead to cusp rupture, perforation, leak, and vegetations. However, if the sewing cuff is involved in infection, the pathologic process is similar to that of mechanical prosthetic valve infection.
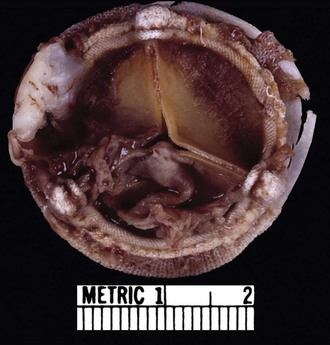
The sizes and types of vegetations appear to be correlated with the virulence of the causative microorganism. S. aureus usually results in smaller vegetations but causes significant destruction and invasion of the tissue. Viridans group streptococci have been associated with larger vegetations with slower, milder destruction of tissue. Fungi form large, bulky vegetations.112 Large vegetations are also associated with HACEK group of organisms.
Clinical Presentation
The clinical presentation of prosthetic valve endocarditis is variable, determined by the virulence of the pathogen and the time of presentation. Common clinical presentations include fever, new/changing murmur, heart block, congestive heart failure (CHF), and embolic events. Fever is the most common symptom and sign of PVE that is present in 73% to 92% of patients. However, fever is often absent in patients with renal failure, elderly patients, and those taking antibiotic or antipyretic medications. New or changing murmur or congestive heart failure may be indicative of complications of PVE such as prosthetic valve dehiscence, fistulas, or perivalvular abscess.113–116 New ventricular dysrhythmias or conduction abnormalities may arise if the infectious process (perivalvular abscess) extends into the electrical conduction system of the heart. Perivalvular extension of the aortic PVE into the intraventricular septum can disrupt the proximal ventricular conduction system and result in complete heart block. Embolic stroke or brain abscess, or both, may be the initial presentation of PVE. In patients with left-sided endocarditis, both symptomatic (35%) and clinically silent (30%) cerebrovascular complications are common.117 Mycotic aneuryms involving the cerebral vasculature are uncommon but serious complications of PVE. The mortality rate associated with ruptured intracranial mycotic aneurysm approaches 80%118–120 and can result in intracranial hemorrhage. Secondary metastatic abscess can develop in the musculoskeletal system (psoas abscess, vertebral osteomyelitis, and diskitis), kidneys, spleen, joints, etc. Right-sided endocarditis involving the tricuspid or pulmonic prosthesis may result in septic pulmonary emboli. Glomerulonephritis or arthritis can be a manifestation of systemic immune response but is uncommon in acute presentations.
Diagnosis
Classic Oslerian manifestations may often be absent in acute PVE because patients present during the early stage of the disease before the evolution of immunologic and embolic manifestations. The diagnosis of prosthetic valve endocarditis can be challenging because atypical presentations are common in the elderly populations with prosthetic valves. High index of suspicion and comprehensive clinical, microbiologic, and echocardiographic evaluations are important for prompt diagnosis of PVE and its complications. Modified Duke criteria should be used as a clinical guide to evaluate all patients suspected to have prosthetic valve endocarditis.121,122 It should be noted that the Duke criteria were established for epidemiologic and clinical research purposes rather than to assist in clinical practice. Hence, clinical judgment should be used when they are applied in the management of patients.
Echocardiography
Transesophageal echocardiography (TEE) is recommended in all cases of suspected PVE.122 One of the two major criteria in the modified Duke diagnostic schema is echocardiographic evidence of oscillating intracardiac mass on the valve or supporting structures, new partial dehiscence of prosthetic valve, or intracardiac abscess.121 The utility of transthoracic echocardiography (TTE) is limited in the evaluation of patients with suspected PVE for two reasons. First, reverberations and other artifacts created by the prosthesis hamper the quality of the TTE images. Secondly, TTE is not sensitive enough to evaluate for perivalvular complications, which is critical in the management of PVE. TEE has a higher sensitivity of 84% to 94% to detect vegetations when compared with TTE (33% to 68%).123–125 TEE is also superior to TTE for the detection of complications such as perforations, prosthetic valve dehiscence, perivalvular abscesses, and fistulas.126 Thus, TEE is important to both establish the diagnosis of PVE and identify high-risk patients who may require early surgery.
False-negative TEE may occur in the early course of illness if the vegetations or perivalvular abscesses are too small to be detected.127 TEE images may be limited if the abscess is localized around calcification in the posterior mitral annulus and in patients with a Bentall procedure (composite graft placement of the aortic valve, aortic root, and ascending aorta, with reimplantation of the coronary arteries into the graft). If the clinical suspicion persists despite negative TEE, TEE should be repeated after 2 to 7 days. In experienced laboratories, the specificity of an abnormal TEE is about 95%. The interpretation of TEE is sometimes difficult because differentiation among thrombus, vegetation, or degenerative strand is not always obvious.
Complications of PVE such as valve dehiscence, perivalvular abscess, fistulas, and pseudoaneurysm may evolve over time despite negative initial TEE. Persistent fever despite appropriate antibiotic treatment and/or new conduction abnormalities should raise the suspicion for intracardiac perivalvular abscess. New or worsening heart failure may suggest mechanical valve dehiscence or perforation/rupture of the bioprosthetic valve cusp. TEE should be repeated promptly whenever there is a change in clinical condition that raises the suspicion of PVE complications. TEE may identify pseudoaneurysms as pulsatile, echocardiographic-free sac around the prosthesis communicating with a cardiac chamber (Fig. 83-5).
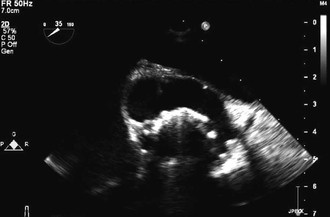
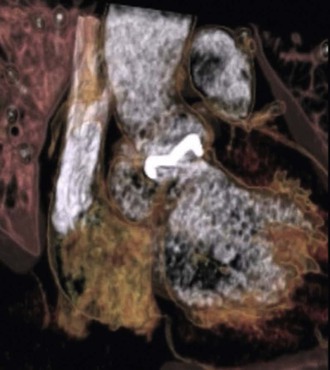
A new imaging modality, multislice computed tomography (Fig. 83-6), was shown in one study to have excellent sensitivity (97%) when compared with TEE and provided more accurate anatomic detail about the perivalvular extent of abscess/pseudoaneuryms than TEE.128 This and other newer tools require additional investigation as to how they should be used in patients with PVE.
Identification of the Pathogen
Blood Cultures
Blood cultures remain the most valuable test in establishing the etiologic diagnosis of PVE. In clinically stable patients, at least three sets of blood cultures should be collected over several hours before the initiation of antibiotics. If the clinical situation is urgent, empirical antibiotic therapy can be initiated after obtaining two or more blood cultures sequentially over a short interval of time. Blood cultures can be obtained at any time and are not necessarily timed with fever or chills because the bacteremia in endocarditis is low grade and continuous.129,130 Growth of the microorganism in blood culture allows us to perform antimicrobial susceptibility testing, which is crucial in the selection of the most effective antibiotic regimen. Recent antibiotic administration can render the blood culture negative,131 and thus it is crucial that blood cultures be drawn before initiation of antibiotics in all patients with suspected PVE.
Blood cultures are positive in about 78% to 91% of patients with PVE.8,14,29,114,132 Advances in bacterial culture techniques, with prolonged incubation times, presence of carbon dioxide, and use of enriched culture media have improved the yield of blood cultures. However, the prevalence of blood “culture-negative” PVE is still significant.14,19 Recent antibiotic administration is the most common cause of blood “culture-negative” PVE. Careful history and documentation of prior antibiotic use will help in the evaluation of patients suspected of culture-negative PVE. Routine blood cultures may also fail to detect fastidious microorganisms such as HACEK, Bartonella, Brucella, Abiotrophia, Granulicatella spp., and noncultivable microorganisms such as Chlamydia spp., Coxiella burnetii, Legionella spp., Mycobacterium spp., Mycoplasma pneumoniae, Tropheryma whipplei, and fungi.131 In these situations, close collaboration between the clinician and laboratory staff is required to determine the appropriate specialized culture media/techniques, serologic tests, and/or molecular tests that can be employed to identify the pathogen.
Valve Culture
Surgical specimens, including vegetations, tissue fragments of bioprosthesis, periprosthetic tissues/abscesses, and embolic fragments, should be sent for histopathology and cultures in patients who undergo surgery. Cultures of the valves are often negative due to prior antibiotic administration.133,134 Valve culture results should be interpreted with caution because contamination during handling from surgical resection to laboratory processing is possible.135–137
Serologic Methods
Serologic methods can be used to complement culture-based methods to identify a pathogen. Modified Duke criteria have included specific serologic data as surrogate markers for positive blood cultures to establish an etiologic diagnosis in “culture-negative” endocarditis. An anti–phase I immunoglobulin G antibody titer of greater than or equal to 1 : 800 by microimmunofluoresence to C. burnetii is a major criterion in the modified Duke criteria for Q fever endocarditis.122 Serologic tests are available for Brucella melitensis, Bartonella spp., Legionella spp., M. pneumoniae, Chlamydia spp., and Aspergillus spp.138
Broad-Range Bacterial rDNA Polymerase Chain Reaction
The amplification of bacterial 16S rDNA using broad-range DNA primers and subsequent nucleotide sequencing can help identify an etiologic agent of PVE and are principally used in culture-negative cases.139 16S bacterial ribosomal RNA (rRNA) genes comprise both highly conserved and variable regions. Using broad-range PCR primers to amplify the conserved region of 16S rRNA genes, it is theoretically possible to identify all possible bacterial pathogens.140 PCR amplification of conserved sequences in the panfungal small-subunit rRNA internal transcribed spacer sequence can identify fungal microorganisms.141
In certain clinical situations, a more accurate identification of the pathogen to the species level may have important clinical implications. For example, in cases of CoNS PVE, it is important to identify Staphylococcus lugdunensis because it has a more virulent clinical course. To identify microorganisms at the subspecies level, the highly variable ribosomal intergenic sequences can be targeted for PCR amplification. In addition, this amplification can be extremely useful in defining whether a pathogen has genes that encode for antimicrobial resistance; this would be helpful in the case of identification of a staphylococcus and whether it is oxacillin resistant, for example.
PCR-based techniques have been used to detect microorganisms in blood specimens, as well as resected heart valve tissue.134,142–151 Molecular techniques have higher sensitivity and specificity than conventional culture-based methods. In one recent study of blood culture–negative PVE, a high prevalence of fungal pathogens was detected using molecular and serologic methods.152 The molecular techniques are especially useful in the context of “culture-negative” endocarditis because the 16S rRNA genes extracted from the valve tissue can help to identify the cultivable but dead organisms (prior antibiotic use) and the fastidious/noncultivable microorganisms. Other genes such as 23S rRNA, 16S-23S intergenic spacer, and rpoB have been successfully used.140
Microbial DNA can be present in the valve tissue for several weeks after the initiation of appropriate antimicrobial agent.147,153 PCR-based methods also do not differentiate between viable, dead microorganism and contaminating free microbial DNA. Hence, positive PCR from the resected valve tissue should be interpreted within the clinical context.
Molecular techniques cannot replace culture-based methods but are additional tools that can be used to improve the etiologic diagnosis of IE. Currently, culture is indispensable for antimicrobial susceptibility testing. Molecular techniques targeting common resistance genes to predict resistance patterns may provide rapid and useful information. Standardization of molecular techniques is necessary to reduce the interlaboratory variations and to reduce contamination because a broad-range 16S rDNA PCR is prone to contamination.
Novel technologies such as DNA microarrays may be useful to provide more accurate and rapid microbial identification.
Histology
Histologic examination of resected perivalvular and valvular tissues remains the “gold standard” for the diagnosis of PVE. Histologic criteria of PVE include demonstration of microorganisms in the resected specimens and pathologic lesions such as vegetations and inflammatory infiltrates, with or without annular abscess.154
In situ visualization of causative agents in the resected valve specimens can be done with immunohistochemical methods. Autoimmunohistochemistry is an immunohistochemical method in which a patient’s own serum is used as a source of antibodies that can be used to detect microorganisms in heart valve specimens.155 Fluorescence in situ hybridization using fluorescent peptide nucleic acid (PNA) probes can penetrate into gram-positive bacteria within the resected valve tissue and can help to visualize the organism in situ.156
If histologic examination fails to demonstrate microorganisms and vegetations, computer-assisted quantitative analysis of digitized microscopic images of the inflammatory patter may assist in differentiating infective and noninfective inflammatory valve processes. Excessive neovascularization and a pattern composed of CD15+ leukocytes exceeding 2% of the mechanical valve surface and exceeding 1.5% of the total bioprosthetic valve surface is highly predictive (90% and 98%, respectively) and specific (90% and 94%, respectively) for an acute infection.154,157
Evaluation of Bloodstream Infection in Patients with a Prosthetic Valve
Certain microorganisms have a high propensity to cause prosthetic valve endocarditis. Among patients with underlying prosthetic valve who develop staphylococcal bacteremia, approximately 50% of patients with S. aureus bacteremia and 40% of patients with CoNS bacteremia have or will develop PVE.35,158 The risk is high even in the absence of persistent fever and persistent bacteremia.159,160 The risk appears to be independent of the type, location, or age of the prosthetic valve.35,158 Among patients with prosthetic valve and enterococcal bacteremia, PVE was observed in 8% to 32%.161 Hence, patients with prosthetic valves who develop S. aureus, CoNS, or enterococcal bacteremia should be aggressively evaluated for PVE. Nosocomial fungemia has a high risk of causing secondary fungal PVE and should be carefully evaluated.
Management
The management of PVE consists of effective pathogen-specific antimicrobial therapy and timely surgical intervention, when indicated. TEE is used to assess prosthetic valve function and vegetation size and to identify perivalvular complications that may require surgical intervention. It should be repeated during antimicrobial therapy when clinically indicated to monitor for development of complications of PVE. TEE is thus important to determine if a patient can be managed with medical therapy alone or if surgical intervention is necessary.
All patients suspected of having PVE should be hospitalized initially for close clinical monitoring. As previously discussed, pathogen identification, usually by isolation from blood cultures, is critical in defining an optimal treatment regimen. Because the infection is life threatening, it is reasonable to initiate empirical antimicrobial therapy targeting the most likely pathogens after obtaining at least three sets of blood cultures, especially in patients with acute-onset PVE and in hemodynamically unstable patients. It is suggested that the empirical antimicrobial regimen be chosen to target the most likely pathogens of PVE, considering the time of onset of PVE and the likely site of acquisition.
Antimicrobial Therapy
In vitro susceptibility testing should be used to define the most effective, pathogen-specific antimicrobial regimen. A microbicidal regimen is preferred for the treatment of PVE. At least two sets of blood cultures should be drawn every 24 to 48 hours until clearance of bloodstream infection is documented.122 No randomized controlled trials have been conducted to establish the optimal duration of antimicrobial treatment. The duration of antimicrobial therapy is a minimum of 6 weeks and should be counted from the day of first negative blood cultures in culture-positive PVE.122 If the resected valve or tissue culture, or both, are positive, it is reasonable to administer an entire course of antimicrobial therapy. In regimens that include multiple agents, they should be administered in temporal proximity to achieve maximal synergistic microbicidal effect.
Staphylococcal Prosthetic Valve Endocarditis
The antimicrobial regimens recommended for S. aureus and coagulase-negative staphylococcal PVE are identical—based mainly on in vitro susceptibility testing (Table 83-1). A triple-drug combination antimicrobial regimen is recommended for the optimal treatment of staphylococcal PVE.122 The principal drug is a cell wall–active agent, chosen on the basis of methicillin susceptibility. Vancomycin is the principal drug of choice for methicillin-resistant organisms, whereas semisynthetic penicillin (nafcillin, oxacillin) should be chosen for methicillin-susceptible organisms. Isolates of Staphylococcus epidermidis with oxacillin minimal inhibitory concentration (MIC) of 0.25 µg/mL or less may be treated with oxacillin or nafcillin. The second drug in combination therapy is rifampin, which is important in the treatment of staphylococcal foreign body–related infections. In vitro studies, animal models of staphylococcal prosthetic device infections, and clinical studies provide evidence supporting the role of rifampin in eradicating staphylococci adherent to prosthetic devices.162–167 Rifampin resistance can easily develop due to mutation of the ribosomal gene responsible for rifampin site of action.166 The probability of this mutation and hence selection of rifampin-resistant strains is high when a large, highly dense, rapidly dividing bacterial population is exposed to ineffective rifampin-containing regimens. Hence, it is reasonable to initiate rifampin only after an effective two-drug combination antistaphylococcal therapy has been administered for at least 2 days. If the Staphylococcus is not susceptible to two other drugs, a single agent can be used for 3 to 5 days before initiation of rifampin. The goal of this strategy is to reduce the number of organisms and hence lower the risk of selection of rifampin-resistant subpopulation. The third drug in the combination regimen is either an aminoglycoside or a fluoroquinolone. Gentamicin is recommended for the initial 2 weeks of therapy, though there are only limited clinical data to support this addition. In case of gentamicin resistance or intolerance to an aminoglycoside, a fluoroquinolone may be used as a substitute if the strain is susceptible.166–168
TABLE 83-1
Therapy for Prosthetic Valve Endocarditis Caused by Staphylococci, Suggested by American Heart Association
| REGIMEN | DOSAGE AND ROUTE | DURATION |
| Methicillin-Susceptible Staphylococci | ||
| Nafcillin or oxacillin* plus | 2 g IV q4h | ≥6 wk |
| Rifampin† plus | 300 mg PO or IV q8h | ≥6 wk |
| Gentamicin‡ | 3 mg/kg IV/IM q24h in 2 or 3 equally divided doses | 2 wk |
| Methicillin-Resistant Staphylococci | ||
| Vancomycin§ plus | 15 mg/kg IV q12h | ≥6 wk |
| Rifampin† plus | 300 mg PO or IV q8h | ≥6 wk |
| Gentamicin‡ | 3 mg/kg per IV/IM in 2 or 3 equally divided doses | 2 wk |
* Penicillin G, 24 million U/24 hr IV, may be used in place of nafcillin or oxacillin if the strain is penicillin susceptible (MIC ≤0.1 µg/mL) and does not produce β-lactamase. Cefazolin 2 g IV q8h may be substituted for nafcillin or oxacillin. Cefazolin can be used in non-immediate-type allergic reaction to penicillin. Consider skin testing for patients with history of immediate-type allergy to penicillin. Vancomycin is recommended only in patients unable to tolerate penicillins and cephalosporins.
† It is recommended to initiate rifampin therapy only after susceptibility results are known and ideally after 2 days of effective combination therapy, in an attempt to reduce the risk of emergence of rifampin resistance.
‡ Gentamicin should be administered in close proximity to vancomycin, nafcillin, or oxacillin to maximize synergy. Renal function and serum gentamicin concentrations should be closely monitored. Goal trough level is <1 µg/mL and peak level (1 hr postdose) is 3-4 µg/mL.
§ Vancomycin dosage should be adjusted to a trough level of 10-15 µg/mL.
Dosages recommended are for patients with normal renal function.
From Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005;111:e394-e434.
In vitro susceptibility testing should be repeated when there is failure of an antimicrobial regimen. Clinical failure with vancomycin therapy for methicillin-resistant S. aureus (MRSA) PVE can occur due to heterogeneous-type VISA (vancomycin-intermediate S. aureus) strains169 and the optimal antimicrobial therapy in such cases is unknown. Clinical experience is limited in the treatment of PVE with newer agents such as high-dose daptomycin and linezolid.170,171,172–175 No cases of PVE treated with telavancin or ceftaroline have been published to date. Staphylococcal PVE is often complicated by perivalvular extension or prosthetic valve dysfunction, requiring surgical intervention for optimal management.
Streptococcal Prosthetic Valve Endocarditis
The antimicrobial regimens recommended by the American Heart Association (AHA) for the treatment of PVE caused by viridans group streptococci, Streptococcus gallolyticus, Streptococcus pneumoniae, or other penicillin-susceptible streptococci are listed in Table 83-2. The primary drug for treatment of streptococcal PVE is either penicillin or ceftriaxone, given for a minimum of 6 weeks. For PVE caused by highly penicillin-susceptible streptococci (MIC ≤0.12 µg/mL), the addition of gentamicin for the initial 2 weeks is optional because combination regimen has not been shown to have superior cure rates compared with β-lactam monotherapy.122 If the strain does not have high-level gentamicin resistance, intravenous once-daily gentamicin may be administered for the initial 2 weeks in patients who can tolerate aminoglycosides. For patients with PVE caused by streptococci with relative or high-level penicillin resistance (MIC >0.12 µg/mL), Gemella, Abiotrophia, or Granulicatella spp., a combination of penicillin or ceftriaxone with gentamicin is recommended for a minimum duration of 6 weeks. It is suggested to continue gentamicin for 6 weeks if the patient can tolerate it without significant nephrotoxicity. Monotherapy with vancomycin for a minimum of 6 weeks is recommended only for patients who cannot tolerate both penicillin and ceftriaxone.
TABLE 83-2
Therapy of Prosthetic Valve Endocarditis Caused by Streptococci as Suggested by American Heart Association
| REGIMEN | DOSAGE AND ROUTE | DURATION | COMMENTS |
| Penicillin-Susceptible (MIC ≤0.12 µg/mL) Strain of Viridans Streptococci, Streptococcus bovis, Streptococcus pneumoniae | |||
| Either aqueous crystalline penicillin G Or | 24 million units/24 hr IV either continuously or in 4-6 equally divided doses | 6 wk | Gentamicin is optional. Combination of penicillin or ceftriaxone with gentamicin has not been shown to be superior to monotherapy. |
| Ceftriaxone | 2 g IV/IM q24h | 6 wk | |
| With or without Gentamicin | 3 mg/kg IM or IV single daily dose | 2 wk | |
| Relatively or Fully Resistant to Penicillin (MIC >0.12 µg/mL) Strain of Streptococcus or Gemella spp., Streptococcus agalactiae, Abiotrophia defectiva, Granulicatella spp. | |||
| Either Aqueous crystalline penicillin G Or | 24 million units/24 hr IV either continuously or in 4-6 equally divided doses | 6 wk | See below for patients with β-lactam allergy* |
| Ceftriaxone Plus | 2 g IV/IM q24h | 6 wk | |
| Gentamicin | 3 mg/kg IM or IV single daily dose | 6 wk | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree