Prostate Needle Biopsy Techniques and Interpretation
Katsuto Shinohara
Viraj A. Master
Thomas Chi
Peter R. Carroll
INTRODUCTION AND HISTORICAL PERSPECTIVE
Prostate cancer continues to be the most commonly diagnosed cancer in men. In 2009, the American Cancer Society estimated that there will be 192,280 newly diagnosed cases and 27,360 deaths from prostate cancer. While prostate cancer is the second leading cause of cancer death among men, the death rate has been gradually decreasing recently. Early, appropriate and efficient detection efforts are likely to lead to improved outcomes of care. Transrectal ultrasound (TRUS) guided needle biopsy of the prostate is the current gold standard for the early detection of prostate cancer.
Prostate biopsy was first described in 1930 when Ferguson successfully obtained cancer cells by aspirating prostate tissue through a transperineally introduced 18-G needle (1). In 1937, Astraldi described the first transrectal core needle biopsy of the prostate (2). Since then, various instruments have been developed for core needle biopsy. Until the 1980s, a prostate biopsy was commonly done using a large caliber needle, such as a Vim-Silverman, or True-cut needle, and was often performed under anesthesia. The biopsy was done either transrectally or transperineally, and was directed only to the palpable abnormality using finger-guidance.
Transrectal ultrasound (TRUS) of the prostate was first reported by Wild and Reid in 1955 and popularized by Watanabe and associates in the early 1970s (3,4). In the late 1980s, TRUS-guided prostate biopsy using an 18-G needle loaded in a spring action device was first introduced (5). Since then, prostate biopsy under ultrasound guidance using an automated device has become a standard procedure. In 1989, Hodge et al. proposed the modern model of performing biopsies of defined areas of the prostate, namely the “sextant” method of prostate biopsy (parasagittal biopsy at the apex, mid-gland and base bilaterally) (6). After the initial introduction of sextant, systematic prostate biopsy, little refinement of the technique was made until, in an editorial, Stamey suggested moving the biopsies more laterally to better sample the anterior horns of the peripheral zone and avoid sampling error (6,7,8). Such recommendations were supported by the meticulous whole-mount analyses of radical prostatectomy specimens, which helped to further delineate the zonal anatomy of the prostate as well as the spatial origin of prostate cancers (9). Thereafter, technological developments that have improved TRUS and its role in prostate cancer detection include an automated spring-loaded prostate biopsy device, multiaxial planar imaging, and a better understanding of prostate zonal anatomy (10,11).
CURRENT TECHNIQUE
Preparation
Generally, no diet restriction is necessary prior to biopsy. A fleet enema is given 1 hour prior to the procedure. This will evacuate rectal contents including gas and fecal matter, which cause interference on ultrasound imaging. Enema also reduces the incidence of infectious complication (12).
Any anticoagulation medications or antiplatelet medications, such as aspirin, nonsteroidal anti-inflammatory agents, Plavix, etc. should be stopped 1 week prior to biopsy. Although this is a common practice, randomized study did not consistently show higher risk of significant bleeding when patients are continuously taking aspirin at the time of biopsy. Recent studies showed no severe bleeding with continuous aspirin, though it was associated with prolonged mild hematuria and rectal bleeding (13,14). Antibiotics, most commonly fluoroquinolones, are given before biopsy and continued for a total of 48 to 72 hours. A recent study by Lindstedt et al. however described that a single dose of ciprofloxacin 750 mg at the time of prostate biopsy achieved equally low septicemia rates—about 1%—compared with a 3-day antibiotic regimen (15). Usage of antibiotics significantly reduces the risk of septicemia (16). If a patient is known to have heart valve disease or is susceptible to endocarditis, appropriate antibiotics, usually ampicillin and gentamicin, should be given according to the American Heart Association’s guidelines for antibiotic prophylaxis (17). Patients with artificial joints or prostheses are also commonly covered with parenteral antibiotics, although there is no specific practice guideline established. Review articles recommend no routine prophylaxis for patients with artificial joints undergoing dental procedure (18,19). High-risk patients, such as those with recent artificial joint placement or patients with a history of prosthesis infection, are advised to receive appropriate antibiotic coverage.
Fluoroquinolones, with their excellent bioavailability in prostatic tissue and broad Gram-negative coverage, have traditionally been the most widely used antibiotic prophylaxis for transrectal prostate biopsy (20). Over the course of the last two decades, increasing resistance of Escherichia coli, the most common pathogen associated with biopsy-related urinary tract infections, has been documented and in 2006 the first case of multiresistant E. coli sepsis resulting from transrectal prostate biopsy was reported (21). Since that time, multiple series have been published documenting sepsis after biopsy due to drug-resistant bacteria, likely related to extensive fluoroquinolone use (22,23,24). This resistance has extended to non-E. coli sources, such as Enterobacter (25,26).
With these growing concerns for resistance, we recommend that antibiotic selection should be made after reviewing local bacterial-resistance profiles. A population of particular concern is the patient who is immunosuppressed, whether from chronic steroid use, autoimmune deficiency, posttransplant medical regimen, or another source. While limited studies have shown no increased risk for infection after transrectal prostate biopsy in a posttransplant population (27), the immunosuppressed have not been reexamined in the era of growing antibiotic resistance related to prostatic biopsy. Therefore, we recommend exercising particular caution for these patients and counsel them on the potentially significant morbidity conferred from resistant infection in the context of immunosuppression. In addition, patients who have received recent antibiotics, within 6 weeks of biopsy, should also be counseled on a possible higher risk of resistant postprocedure infection.
Transrectal Ultrasonography
Transrectal ultrasonography not only guides the needle into the designated area of the prostate, but also gives information regarding prostate size and shape, tumor localization and stage, and seminal vesicle pathology. Currently, either a biplane probe or end-fire probe with multi frequency between 5 and 10 MHz is used for transrectal prostate biopsy. Depending on the type of probe, the images obtained are slightly different, and sonographers must be familiar with the differences. Details regarding prostatic ultrasound are covered more extensively in Chapter 11.
Biopsy Device
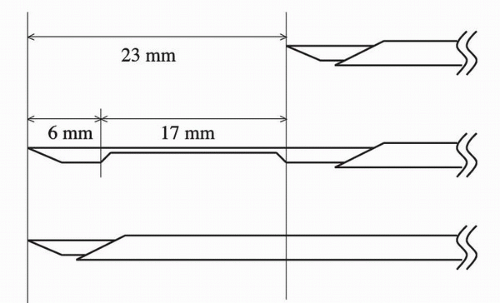
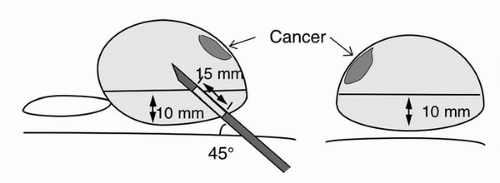
For prostate biopsy, an 18-G needle loaded in a spring action automated biopsy device is commonly used. When the trigger button of this device is pushed, the inner needle advances 23 mm, followed by the outer needle advancing the same distance. The prostate tissue is caught between the inner and outer needle. It is designed to obtain about a 15 to 17 mm length of tissue starting from the tip of the needle before device activation (Fig. 7.1). Therefore, the needle tip must be right at the target before the activation of the dsevice. On transrectal ultrasonography, a needle tract may appear longer than what the biopsy specimen is actually taken. For example, cancer is frequently located along the capsule of the gland. When the anterior biopsy is performed, the needle tip must be advanced into the prostate tissue about 15 mm from the anterior capsule in order to sample the tissue where cancer is frequently located, along the periphery of the gland.
Anesthesia
The administration of anesthetic agents before TRUS biopsy reduces patient discomfort. This may be important as a study from the Netherlands examining the reasons for men refusing to attend a prostate cancer screening showed that 18% of men felt that the prostate biopsy would be painful (28). Nash et al. at UCSF first conducted a randomized, double-blinded study of local infiltration of 1% lidocaine around the prostate vascular pedicle in 64 patients (29). Mean pain scores were significantly lower on the side injected with lidocaine as compared to the saline control side. Many investigators have shown the benefit of the use of anesthetic techniques (topical, injected or both), although there are a few studies where a benefit has not been shown (30,31,32,33).
The previously described technique of injecting lidocaine at the vascular pedicle can be associated with systemic circulation of lidocaine, which may result in a sensation of bad taste, ear tingling, or vertigo. In addition, this type of nerve block does not completely anesthetize the anterior region of the gland. Currently at University of California, San Francisco (UCSF), a 1% lidocaine 20 mL and 7.5% sodium bicarbonate 2 mL solution mixture is used to reduce the burning sensation associated with injection and anesthetize the gland. The solution is injected directly into the prostate at three locations in each lobe by inserting a 22-G needle all the way to the anterior capsule at the base, mid gland and apex. As the needle is pulled back, 1 to 2 mL of anesthetic is infiltrated into the prostate parenchyma at each location. By doing this, systemic circulation of anesthetic can be avoided, and the entire gland is anesthetized, including the anterior region. Mutaguchi et al. compared this technique with conventional nerve block and concluded superiority in anesthetic effect utilizing intraprostatic injection (34).
Issa et al. reported the benefit of 2% lidocaine jelly injected into the rectum prior to biopsy (35). Pain associated with biopsy generally originates from the prostate capsule and parenchyma, and not from the rectal wall. Sensation of the ultrasound device being in the anus is another component of discomfort during the biopsy. Anesthetic jelly generally anesthetizes the anal ring and proctocanal, achieving a more comfortable biopsy procedure. However, intrarectal injection of anesthetic jelly alone does not completely block the pain due to needle penetration in the prostate. Some anesthetic effect of the proctocanal with lidocaine jelly can be achieved by 20% benzocaine cream (Hurricaine, Beutlich Phamaceuticals, Waukegan, IL) applied at the time of the digital rectal examination prior to biopsy. Benzocaine is a faster acting topical anesthetic achieving effective anesthesia of the mucosa in 30 seconds. Combining both intrarectal anesthesia and intraprostatic block appears to provide maximum comfort during prostate biopsy (36).
Procedure
Positioning
The left lateral decubitus position is commonly preferred for transrectal prostate biopsy. The patient’s buttock should be placed on the closer edge of table to the operator. The hip and knee joints are flexed to a right angle and the legs are placed along the far edge of the table from the operator in order to create a wide area below the buttocks for maneuvering the TRUS probe and biopsy device. The pelvis should be slightly tilted forward. This will also allow more freedom of probe movement during biopsy.
Location and number of biopsies
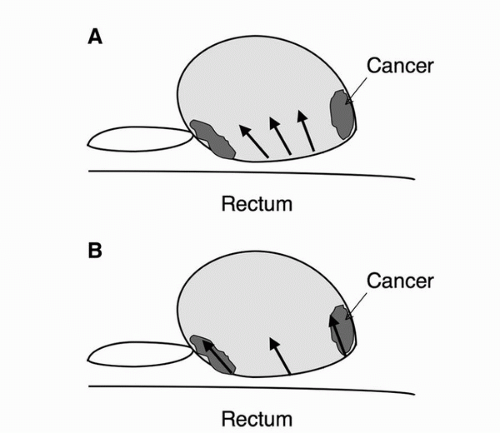
Many urologists, both in academic and community practice, utilize an extended sextant biopsy technique, obtaining two specimens from each sextant, resulting in a 12-core sample acquisition (37,38). Extended-pattern biopsy techniques are associated with improved cancer detection rates compare to the standard sextant (six core) technique (37). For an extended sextant biopsy, within each sextant, one biopsy is obtained medially, close to the median furrow of the gland and the other laterally from the region close to the lateral edge of the gland. At UCSF, in addition to extended sextant biopsies, a pair of samples along the anterior capsule of the apex/mid gland is routinely performed (Fig. 7.2). Lesiondirected biopsies are routinely performed as well. Using such a scheme, the cancer detection rate at UCSF and most other centers approximates 44%. Biopsy results stratified according to serum PSA ranges are shown in Table 7.1. Each biopsy entry site has to be separated well in order to cover the gland widely (Fig. 7.3B). However, a common technical error is that biopsies targeting the apex and base of the gland are both obtained close to the mid-portion of the gland (Fig. 7.3A). Such biopsy technique results in samples originating from almost the same area of the prostate rather than distinct regions of the apex, mid-portion, and base.
When the needle is advanced through the biopsy guide, the first sensation of resistance one encounters is from the rectal wall. With further advancement of the needle, the second resistance encountered is from the prostate capsule. At this point, TRUS imaging should show the needle tip to be slightly indenting the prostate capsule. This confirms appropriate depth of positioning for the needle tip. If the needle tip is not in contact with the capsule or the tip is already in the prostate parenchyma, satisfactory biopsy sampling cannot be achieved.
When the very apex of the prostate is biopsied, the needle path may puncture the proctocanal distal to the dentate line. In such cases, a patient may experience significant discomfort, because the mucosa distal to the dentate line is very sensitive to pain. The needle tip should instead be advanced above the dentate line, and then redirected to the apex within the rectum in order to avoid such pain. Some ultrasound probes are designed with the biopsy guide traversing through the middle of the probe itself. Such probes have less flexibility for maneuvering the biopsy direction, since the angle of the biopsy guide to the prostate is almost fixed. Apical biopsy technique as described above with this type of probe can be challenging, because it is more difficult to redirect the needle tip away from the proctocanal distal to the dentate line using these probes compared to using an end-fire probe with the needle guide attached to the side of the probe. It is important to note that biopsy of the base or anterior part of the gland frequently results in the needle penetrating the bladder wall, since the needle advances a total of 23 mm. If the bladder wall is penetrated, ultrasonography can show the severity of the bleeding from the bladder wall. If continuous pulsatile blood is noted at the end of the procedure, an insertion of a Foley catheter should be considered in order to monitor and stop further bleeding.
TABLE 7.1 BIOPSY RESULTS WITH EXTENDED PLUS 2 ANTERIOR BIOPSY SCHEME AT UCSF | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
In certain circumstances, the use of finger-guided transrectal prostate biopsy can be of value. When a palpable nodule has no corresponding visible lesion on ultrasound imaging, fingerguided biopsy can offer the ability to achieve lesion-directed biopsies. When comparing efficacy of finger-guided prostate biopsies to ultrasound-guided biopsies, Abrams et al. found no difference in detection rates regardless of the presence of a palpable lesion with the use of systematic, multiple finger-guided biopsies (39). Chiang et al. demonstrated that finger guidance and ultrasound guidance yielded similar detection rates in the presence of a palpable nodule and combining the two techniques increased the sensitivity of detection (40). This technique can be performed safely by placing a shortened biopsy needle sheath against the pad of the gloved index finger and placing a second glove over the sheath and gloved hand, thereby sandwiching the sheath between the two glove layers. The index finger can then be used to palpate the prostate nodule after which
the prostate biopsy needle is advanced through the sheath into position and activated (41). With this technique, the operator finger is protected from direct contact with the needle tip using the sheath and the needle is well controlled.
the prostate biopsy needle is advanced through the sheath into position and activated (41). With this technique, the operator finger is protected from direct contact with the needle tip using the sheath and the needle is well controlled.
Transition Zone Biopsy
According to Hodge and McNeal et al., prostate cancer frequently originates from the peripheral zone, but about 24% of cancer arises from the transition zone (6). Chang et al. evaluated the role of a routine transition zone biopsy in patients with a large prostate gland, defined as more than 50 mL, and concluded that there is little benefit of adding transition zone biopsy in such patients (42). Takashima et al. carefully examined radical prostatectomy specimens in 62 T1c cancer patients and reported that T1c cancer is densely located in the apex to mid portion of the anterior half of the gland (43). Prostate cancer is often times not detected without adequate sampling of the anterior region of the gland, as this is an area of the prostate prone to cancer growth and easy to miss with the extended sextant biopsy. Based on these data, at UCSF, we routinely biopsy the anterior region of the prostate, and not the transition zone, in addition to the extended sextant biopsies. With this biopsy scheme, two anterior biopsies are obtained from the peripheral zone located anterior to the urethra at the apex and extending anteriorly along the lateral edge of the mid gland. The positioning of the needle is illustrated in Figure 7.2 again. This figure demonstrates the location of anterior prostate cancer in red which can be missed without the addition of anteriorly directed biopsies. Meng et al. reported that adding anterior apical biopsies to the extended sextant biopsy scheme increased cancer detection, especially in patients with a normal digital rectal examination (44). These anterior samples are thus more likely to increase cancer detection compared to transition zone samples. When the anterior part of the gland is biopsied, needle tip must be located 15 mm from the anterior capsule in order to sample the tissue from the prostate parenchyma at the needle tip all the way up to the anterior capsule when the biopsy device is activated.
REPEAT BIOPSY
With conventional TRUS technology, sampling errors are inevitable and a negative biopsy does not rule out malignancy with certainty. Management of the patient with repeatedly negative prostate biopsies and clinical characteristics suggestive of cancer, such as an elevated PSA or abnormal digital rectal exam remains a challenging problem for physicians and patients. Repeat prostate biopsies will detect cancer in 16% to 41% of cases in which an initial biopsy was negative (37). At UCSF, Shinohara et al. attempted to identify predictors of positive biopsy in order be able to avoid unnecessary biopsy procedures in patients at low risk for malignancy, studying 325 men with a history of two or more negative biopsies. The mean age of this patient population was 61 years with a mean serum PSA of 13.8. The repeat, positive biopsy rate in these patients was 38%. The percentage of patients with a positive biopsy decreased as the number of previous negative biopsies increased: 40% in patients with two to three prior negative biopsies, 36% in patients with four to five prior negative biopsies, and 17% in patients with six prior negative biopsies. Using a Cox proportional hazards model, predictors of positive biopsy were identified including higher serum PSA, increased age, hypoechoic lesions on ultrasound, and a smaller prostate. Interestingly, abnormal pathology (prostatic intraepithelial neoplasia [PIN], atypical small acinar proliferation [ASAP]) on previous biopsy, abnormal digital rectal exam, and transition zone volume were not significant predictors of a positive biopsy (unpublished data).
Repeat Biopsy Technique
When performing repeat biopsy, the standard parasagittal loci, lateral mid-prostate and base, anterior apex and transition zones should be sampled. Additional needle biopsies should be obtained from the site of any initial high-grade prostatic intraepithelial neoplasia (HGPIN) or ASAP, as well as any suspicious lesions visualized on ultrasonography. Finger-guided prostate biopsy can be considered for sample any nodules not visible on ultrasound imaging. The most common areas where prostate cancer can be missed on initial biopsy are at the apices as well as in the anterior prostate (see Fig. 7.2 for illustration of anterior lesion location). In those undergoing repeat biopsy, additional biopsies should be directed to areas not previously sampled, such as the very apex, very lateral edges, midline, and along the anterior capsule. Recently, a series of TRUS guided transperineal template prostate biopsies showed significant apico-anterior cancer detection in patients with a normal digital rectal examination (45). The transperineal approach is therefore favored by some for secondary/repeat biopsies. With a transperineal technique, the anterior and apical gland can be systematically sampled more thoroughly compared to the transrectal approach due to the direction of needle insertion (Fig. 7.4). See the section below on transperineal template prostate biopsy for further elaboration.
Strategy for High-Grade PIN and Atypical Small Acinar Proliferation
HGPIN is found on a varying, but significant fraction of prostate biopsies (1%-25%), with most modern series demonstrating an average of 5% of all biopsies (46). PIN is characterized by architecturally begin prostate acini which are lined by cytologsically atypical cells. HGPIN may be a precursor lesion to adenocarcinoma (47,48). The discovery of HGPIN on first prostate biopsy usually prompts repeat prostate biopsy in 3 to 6 months. Published series of those with HGPIN who undergo repeat biopsies show a subsequent cancer detection rate of 30% to 50%. However, Lefkowitz et al. reported that with extended biopsy scheme showing HGPIN, repeat biopsy showed cancer in only 2.3% of cases. They recommended that immediate repeat biopsy is not necessary after 12-core biopsy showing HGPIN (49).
ASAP, which has also previously been termed atypical adenomatous hyperplasia or atypia, is characterized by the crowding and proliferation of small glands; however cytological atypia is minimal (50). This lesion has been less well characterized than HGPIN, but ASAP alone is identified in 5% of patients undergoing needle biopsy. Iczkowski et al. proposed further classification of this lesion into three
categories—favor benign, uncertain, and favor malignant— and suggested correlation of each category to subsequent cancer detection (51). Importantly, the association of ASAP with prostate cancer is higher than that of HGPIN. Contemporary biopsy series looking at the influence of ASAP have shown the probability of detecting adenocarcinoma on repeat biopsy is 40% to 50%. Having both HGPIN and atypia together on the first biopsy may increase the rate of cancer detection on the second biopsy to as high as 75% (52). In one study by Park et al., between 1991 and 1998, 45 men with atypia underwent rebiopsy and 23/45 (51%) were found to have cancer. Ninety one percent of these patients had a positive biopsy for cancer on their repeat biopsy, while 9% required two or greater biopsy sessions (53). Cancer is more likely to be detected in the sextant where HGPIN or ASAP was found.
categories—favor benign, uncertain, and favor malignant— and suggested correlation of each category to subsequent cancer detection (51). Importantly, the association of ASAP with prostate cancer is higher than that of HGPIN. Contemporary biopsy series looking at the influence of ASAP have shown the probability of detecting adenocarcinoma on repeat biopsy is 40% to 50%. Having both HGPIN and atypia together on the first biopsy may increase the rate of cancer detection on the second biopsy to as high as 75% (52). In one study by Park et al., between 1991 and 1998, 45 men with atypia underwent rebiopsy and 23/45 (51%) were found to have cancer. Ninety one percent of these patients had a positive biopsy for cancer on their repeat biopsy, while 9% required two or greater biopsy sessions (53). Cancer is more likely to be detected in the sextant where HGPIN or ASAP was found.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree