Key points
- •
Properly identify the indications and goals for the procedure to guide selection of embolic material.
- •
Become familiar with available embolic agents and common uses for each.
- •
A clear understanding of the relevant anatomy will allow for a safe and efficient embolization.
Introduction
Transcatheter and percutaneous embolization therapies are a large part of the Interventional Radiologists’ armamentarium. Embolotherapy has a wide array of applications such as control of bleeding in a variety of settings, tumor therapy, and treatment of certain vascular abnormalities. In this chapter, we provide a brief overview of the different embolic agents currently available. The choice of agent must be made according to the appropriate clinical scenario, which can be varied. For example, compare the following two patients: the first being one who is involved in a motor vehicle accident sustaining solid organ injury with evidence of active arterial bleeding on imaging; the second, a consultation regarding nonsurgical treatment options for a liver tumor. Both can be treated with transcatheter embolization; however, because of the different clinical scenarios, the approach to each, risks of embolization, and the choice of embolic agent are all radically different. Some key factors that should be considered include the following:
- 1.
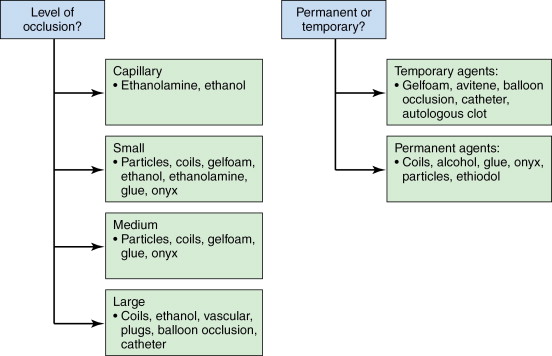
What is the goal of the procedure? Is temporary or permanent occlusion required?
- 2.
What is the desired level of occlusion?
- 3.
What is the relevant anatomy? How does this influence the risks of nontarget embolization?
- 4.
What is the appropriate delivery system?
In this chapter, we will provide an overview of the most commonly employed embolic agents and their typical uses. We divide embolic agents into two major categories: temporary versus permanent. The temporary agents are commonly used in the setting of trauma or times when a permanent occlusion may result in unacceptable end-organ damage. Gelfoam is the prototypical temporary agent and is versatile in both its uses and methods of deployment.
Among the permanent embolic agents, we first discuss particles such as polyvinyl alcohol (PVA) and acrylic spheres, which are available in a number of different size ranges and commonly used in tumor and solid organ embolization procedures. Coils are another commonly used embolic agent and are available in a variety of sizes to occlude small, medium, and large vessels. Some common uses for coils include treatment of pseudoaneurysms, blockage of collateral vessels during Y-90 treatments, and treatment of pulmonary arteriovenous malformations (AVMs). Finally, we will review some commonly employed sclerosants and glues, frequently used for the treatment of peripheral vascular malformations. Figure 1-1 provides a brief overview of the most commonly employed embolic agents.
Temporary agents
Gelfoam
Gelfoam is a water-insoluble hemostatic gelatin sponge that expands on contact with fluid. A gelatin derived from purified porcine skin, gelfoam is a flow-directed embolic agent used for temporary vascular occlusion that induces hemostasis by promoting platelet aggregation, vessel wall inflammation, and thrombus formation. As a temporary agent, it is most useful in the setting of trauma to control bleeding. Gelfoam is available as sterile sheets of varying thicknesses that can be cut into smaller pieces or as a fine powder, approximately 50 μm in size. Care must be taken when used in the powder form as occlusion is often permanent because of the small size of the particles, and can result in tissue necrosis. Gelfoam sheets are versatile and can also be prepared in a slurry by cutting or shaving small strips of the gelfoam and loading them into a syringe mixed with contrast and/or saline.
It has been observed histologically that within 6 days of gelfoam embolization, an acute inflammatory and foreign body reaction occurs as characterized by giant cells followed by an inflammatory cascade resulting in thrombus formation. Recanalization has been estimated to be anywhere from 3 weeks to 4 months in animal studies with vessel recanalization in two patients undergoing embolization for renal cell carcinoma occurring in 5 and 6 months. , It must be reiterated though that permanent gelfoam occlusion has also been demonstrated and is thought to be secondary to aggressive and densely packed gelfoam.
Avitene
Avitene (Davol Inc., Warwick RI) is a microfibrillar collagen preparation that comes in a variety of forms including powder, sheets, and sponges. The uses and preparation are similar to that described for gelfoam although it is not as commonly used. Although considered a temporary agent with vessel recanalization in a few weeks, this agent causes an intense inflammatory reaction with immediate thrombosis in large and small vessels, which may be permanent in some cases.
Permanent agents
Particles
Polyvinyl alcohol
PVA particles are derived from shavings of blocks of inert plastic (polyvinyl alcohol) sponges. They are irregular in size and shape and come in a variety of differing size ranges from 50 to 2500 μm. PVA is injected via a catheter to occlude small arteries and arterioles by both a mechanical occlusion and creation of an inflammatory reaction in the vessel wall that leads to fibrosis, thrombosis, and occlusion. Because the particles expand on contact with fluid and tend to clump together, there is a tendency for this embolic to produce a more proximal blockade in comparison to uniformly sized particles. Albumin can be added to the suspension to help prevent clumping of these irregular particles.
As a permanent embolic agent that is available in a variety of different sizes, PVA is well suited for a variety of procedures, including tumor embolization. As with other particles, the operator must be aware of the risk of reflux and nontarget embolization. Also, very dense solutions of PVA can cause clumping of the particles, leading to catheter occlusion or proximal vessel occlusion. Angiographically, this may appear to be a successful embolization with cessation of flow to the target area. However, the particles that had clumped together may potentially break down later, leading to an incomplete embolization. ,
Acrylic spheres
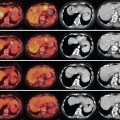
Tris-acryl spheres are particles that are uniform in size and shape that can be cross-linked to gelatin. The spheres are hydrophilic and designed to be non-aggregating to deliver a more predictable distribution. The soft, elastic nature of these spheres allows for compressibility of the particles by up to 33% in diameter. This reduces the problem of catheter clogging that can be found with PVA, and allows for more distal penetration in the vascular bed as compared to similarly sized PVA. , Once ejected from the delivery catheter, the particles are carried to their eventual point of occlusion via arterial blood flow around the delivery catheter. In this manner, hypervascular tumors are preferentially embolized. The compressibility and nonaggregating nature of the particles result in a more distal vessel occlusion compared to similarly sized particles of another material. Because the particles occlude a more distal vascular bed, there are higher rates of infarction. Knowledge of the appropriate-size particle to utilize for each tumor type is important because if incorrectly sized, there are risks of escape to the systemic circulation via arteriovenous shunts. , , This agent is available from various manufacturers. Embosphere particles (BioSphere Medical, South Jordan, UT) are available in 1–2 mL aliquots suspended in sterile saline and in the following size distributions ( Figure 1-2 ):
- •
40–120 μm
- •
100–300 μm
- •
300–500 μm
- •
500–700 μm
- •
700–900 μm
- •
900–1200 μm

Embozene ® Microspheres (CeloNova BioSciences, San Antonio, TX) is another particle embolic that offers microspheres that are color-coded by individual particle size, which ranges from 40 to 1300 μm.
Other particle embolics are also in the market such as Bead Block (Biocompatibles, Surrey, United Kingdom), which represents a spherical embolic that is derived from a PVA hydrogel. There is tremendous interest in determining the relative efficacy of each of these embolic choices. , ,
One of the major risks of embolization with particles is nontarget embolization to vital structures. A tool for minimizing the risk of such nontarget embolization is the balloon occlusion catheter. , These catheters are designed to enhance the safety of the delivery of embolic agents to an intended target by minimizing or eliminating the risk of reflux around the catheter to nontarget sites. This end-hole catheter, commonly designed with a balloon set 1 cm from its tip, can be inflated to temporarily occlude the vessel feeding the target. Once this vessel is occluded, the embolic material can be injected. One drawback with such a design is that total vessel occlusion does not allow for antegrade blood flow, which would limit the ability of controlling the amount of embolic agent reaching the intended target. Novel designs of some newer antireflux catheters are able to circumvent this issue (Surefire Medical, Inc., Westminster, CO). Such antireflux catheters make use of an expandable, thin-walled, funnel-shaped tip that is designed to expand and occlude the vessel during retrograde flow and prevent particle reflux. The funneled tip is designed to collapse during antegrade flow, thus allowing for delivery of the embolic agent to the intended target.
When performing an embolization procedure using particles, there is a degree of variability among different operators in the exact details; however, the following steps are helpful to keep in mind. Many operators will allow the particles to settle in the syringe and decant most of the saline, replacing the decanted volume with 5–10 mL of contrast to allow maximal visualization during embolization.
The endpoint of the procedure will depend on the goal of the task at hand and is a point of variability among operators. In some instances, the goal may be to continue embolization until there is stasis within the vessel. The definition of stasis in the vessel is open to interpretation, though many consider stasis as persistent contrast visualization in the feeding vessel that persists for 5 cardiac beats. Another accepted endpoint is to continue instilling embolic particles until tumor vascularity is no longer seen, yet maintaining persistent antegrade flow in the parent vessel.
The further out the embolization is carried, the greater will be the degree of stasis in the feeding vessel, thus increasing the risk reflux and nontarget embolization. When approaching the endpoint of the embolization, the operator should flush the volume of the catheter with saline to discard any unnecessary particles. Contrast can then be injected via the delivery catheter to assess the degree of progress.
Transarterial embolization versus chemoembolization
Although a detailed discussion of the differences between transarterial “bland” embolization (TAE) and chemoembolization (TACE) is beyond the scope of this chapter, it is important to understand the basic differences between these techniques.
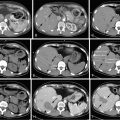
Transarterial embolization, otherwise known as “bland” embolization, refers to the administration of embolic material without delivery of chemotherapeutic agents ( Figure 1-3 ), whereas transarterial chemoembolization refers to the concomitant administration of an embolic agent and a chemotherapeutic drug. TACE can in turn be subdivided into conventional TACE (cTACE) and drug-eluting bead TACE (DEB-TACE). The former is formally defined as the delivery of an agent, most commonly doxorubicin, cisplatin, and/or mitomycin C with or without lipiodol, followed by or coadministered with an embolic agent (either gelfoam or particles). DEB-TACE refers to the procedure by which beads loaded with doxorubicin are transarterially administered.
The premise of transarterial treatment of hepatocellular carcinoma (HCC), for example, is that hepatomas derive the majority of their blood supply from the arterial supply to the liver, whereas the rest of the liver derives most of its blood supply from the portal vein. This unique property of HCC prompted the development of intraarterial chemotherapy and arterial embolization to (1) concentrate chemotherapeutic agents in the tumor and (2) cause selective ischemic necrosis of the arterially supplied tumor. Although these principles appear simple on initial examination, differing practice techniques over the past several decades have sparked a debate as to which of these mechanisms plays a more important role.
TAE works by causing occlusion of the terminal tumor arterioles, leading to ischemic necrosis and cell death. Although tumor necrosis has been demonstrated in several papers, some have argued that tumor hypoxia may actually stimulate angiogenesis and incite mechanisms to resist apoptosis, leading them to question the use of embolization alone in the treatment of HCC. , The counterargument is that more distal embolization is associated with less vascular recruitment and angiogenesis than proximal embolization. This debate has been fueled by the lack of standardization of the size of embolic material used; agents have ranged from 40-μm microspheres to gelfoam cubes.
Conventional TACE aims to concentrate cytotoxic drugs within the tumor and prevent systemic toxicity by using a combination of lipiodol and embolic material. Lipiodol is iodinated poppy seed oil, and it has several unique properties that support its use such as selective uptake in target tumors; slowing the release of cytotoxic drugs it is mixed with; and when used in the liver, is thought to pass through hepatic sinusoids into portal venules, leading to dual embolization of the tumor. ,
Once the combination of lipiodol and cytotoxic drugs is infused, embolic material (e.g., gelfoam, PVA, and tris-acryl microspheres) is administered into the same artery, with the goals of preventing washout of the lipiodol/chemotherapy infusion and causing ischemic necrosis.
DEB-TACE has evolved to improve the pharmacokinetic profile of chemoembolization. In spite of similar quantities of drug infused, drug-eluting beads result in lower systemic doses of doxorubicin compared with conventional TACE. These beads lodge in the tumor microcirculation and elute the loaded drug into the tumor over time. In an animal model, 43% of the drug eluted off the beads after 28 days and ∼90% after 90 days. Drug was detected as far as 600 μm from the bead, and there was a 30% decrease in doxorubicin tissue concentration from 1 to 3 months.
The most commonly used beads are either made of PVA (DC beads, Biocompatibles) or a sodium acrylate–polyvinyl alcohol copolymer (Quadrasphere, Biosphere Medical). These beads range in size from 40 to 900 μm. It should be noted that as of this writing, the United States Food and Drug Administration (FDA) has not approved these beads for drug delivery.
Beads are loaded with doxorubicin in the pharmacy. In the case of DC beads, an ion-exchange mechanism loads oppositely charged doxorubicin onto the beads at a maximum of 45 mg doxorubicin per milliliter of beads, irrespective of bead size. When smaller and larger beads were compared, higher plasma concentrations of doxorubicin were seen with the smaller beads, a finding that was attributed to a higher surface area–volume ratio of the smaller beads, leading to a more robust release of the drug. Quadraspheres expand when hydrated, leading to a final diameter of 200–800 μm. They can be loaded with either doxorubicin or cisplatin, and have CE Mark approval for the treatment of unresectable HCC when loaded with doxorubicin.
A preloaded microsphere (Precision Bead, Biocompatibles) has also been developed. This PVA polymer hydrogel with a sulfonic acid component has 37.5 mg of doxorubicin per milliliter of beads, and ranges in diameter from 100 to 900 μm.
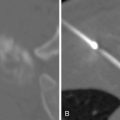
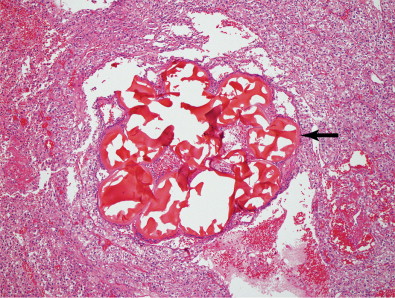
Coils
Coils are thin metal wires that are used for permanent vessel occlusion in a variety of vessels sizes, generally at the pre-arteriolar level. Coils are most often stainless steel or platinum, although platinum coils are preferred because of their increased radiopacity and thrombogenicity. Coils cause vascular occlusion in part by mechanical obstruction, but mainly by inducing thrombus in the vessel. It is important to allow sufficient time for thrombus to form in between coil deployment. Often, a gelfoam slurry can be used in addition to coils to hasten thrombus formation and provide a more complete embolization. Most coils are manufactured with tiny thrombogenic synthetic fibers that are attached to promote clot formation. Coils are available in a variety of shapes, and once deployed in the vessel will try to reassume this shape to help facilitate filling of the entire vessel volume. An example of one such embolic is shown in Figure 1-4 .