Abstract
This chapter reviews 20 years of randomized, prospective clinical research on the use of selective hormone receptor modulators and aromatase inhibitors for the reduction of breast cancer risk in high-risk women. The findings from the trials are summarized using data from the individual trials themselves and published meta-analyses. Also included are recommendations from both US national organizations and international consensus groups that outline the approved agents for specific patients to reduce women’s risk of breast cancer. Data are also included for carriers of predisposing genetic mutations.
Keywords
breast cancer risk, chemoprevention, selective estrogen receptor modulators (SERMs), aromatase inhibitors, tamoxifen, raloxifene, exemestane, anastrozole, atypical hyperplasia, BRCA1 , BRCA2
Identifying Women at Risk
Chemoprevention can be defined as the use of natural or synthetic chemical agents to reverse, suppress, or prevent carcinogenic progression to invasive cancer. Epidemiologic data suggesting that breast cancer is preventable through drug intervention include time trends in cancer incidence and mortality, geographic variations and the effects of migration, identification of specific causative factors, and the observation that most human cancers do not show simple patterns of genetic inheritance.
Chemoprevention may be recommended for certain women who are at increased risk of breast cancer. Indeed, the need for effective breast cancer preventive strategies is apparent based solely on the number of women who are at increased risk for the disease. More than 45 million women in the United States are older than 50 years of age, and at least 2.5 million of these women have first-degree relatives with breast cancer. At least 8 million postmenopausal women have undergone biopsy for benign breast disease, and one in four of these women have proliferative changes. At least 10 million older women are obese, and one in six women 40 years of age or older is nulliparous. A substantial proportion of breast cancer occurs in women with these characteristics, and strategies to reduce this risk would have a significant effect on the burden of breast cancer in the United States.
The clinician’s role in identifying candidates for chemoprophylaxis should include a detailed assessment of familial breast cancer, the opportunity for genetic testing when appropriate, comprehensive quantitative risk assessment, and a specific management prescription. Clinicians should also address the risks and benefits of screening, prophylactic surgery when indicated, and risk reduction using approved chemopreventive agents.
Familial breast cancer—often in a mother, aunt, and/or sister—is a leading reason why women seek counseling from their physicians about their own risks of developing breast cancer. Well-characterized breast cancer susceptibility genes, including BRCA1 and BRCA2, may account for as many as one in six breast cancers. The cumulative lifetime risk of developing breast cancer approaches 85% for some carriers of BRCA mutations, as estimated by the number of relatives with positive breast diagnoses before 50 years of age. If there is a suspicion that one of the susceptibility genes may be involved in the etiology of the breast cancers in a woman’s family, further risk assessment is recommended.
Clinicians should strive to ensure that the patient understands her objective risk and its implications for making a decision about chemoprevention. In addition to genetic susceptibility, hormonally linked adult reproductive and anthropometric risk factors have been well established in the etiology of pre- and postmenopausal breast cancers, and early-life exposures have also been evaluated.
Breast Cancer Risk Models
Multiple quantitative models are available to assess a woman’s risk of developing invasive breast cancer, but all of the models are limited by moderate discriminatory accuracy. The quantitative breast cancer risk assessment model developed by Gail and colleagues estimates the probability that a woman who engages in annual mammographic screening will develop invasive or in situ ductal or lobular cancer over a particular age interval. The model has been widely used in clinical trials of pharmacologic agents to reduce the risk of breast cancer. There are six risk factors in the model, and they were adjusted simultaneously for the presence of the other risk factors. They include current age, age at menarche, number of breast biopsies, age at first live birth (or nulliparity), family history of breast cancer in first-degree relatives, and race. Risk of breast cancer may be determined by validated models other than the Gail model (including the Tyrer-Cuzick model ) or by the eligibility criteria used in the various breast cancer chemoprevention trials.
The Gail model is available online at www.cancer.gov/bcrisktool . The average American woman’s Gail score is 0.3%, which represents her estimated risk of developing invasive breast cancer over the next 5 years; the lifetime risk for the average American woman is 10.1%. A previous diagnosis of atypical lobular or ductal hyperplasia nearly doubles the estimated risk, although the model underestimates the risk of women with atypical hyperplasia. The model has high predictive accuracy within populations or among groups of women, and it estimates the absolute risk of developing invasive breast cancer only in women 35 years and older. It was not designed for women with prior diagnoses of breast cancer, lobular carcinoma in situ (LCIS), or ductal carcinoma in situ (DCIS).
The Breast Cancer Risk Assessment tool has validity for estimating risk in African American women. It represents one of the easiest, least expensive, and enduring ways to assess objectively those women who are at greatest risk of developing breast cancer. The tool and model is only appropriate for breast cancer risk estimation in high-risk women who do not present with genetic susceptibility genes.
The 5-year risk has been the standard used for decision-making about chemoprevention because the National Cancer Institute (NCI) breast cancer risk assessment tool, which reports a 5-year risk for invasive breast cancer, was the basis for enrollment onto the two major US prevention trials and forms the basis of the US Food and Drug Administration (FDA) indication for both tamoxifen and raloxifene. The US Preventive Services Task Force (USPSTF) guidelines (discussed later in the chapter) state that, for women with a 5-year risk 3% or greater, and using models such as the NCI breast cancer risk assessment tool, a provider should discuss the use of selective estrogen receptor (ER) modulators (SERMs) for primary prevention. Published estimates show that 27% of women with proliferative benign breast disease have an estimated 5-year risk 3% or greater.
Mammographic Density
The extent of hormonally active and proliferative breast tissue is positively associated with increased breast cancer risk. Women with dense tissue in at least 75% of their breasts have a risk of breast cancer four to six times as high as women with low or normal breast densities. The associations between mammographic density and both DCIS and invasive breast cancer are similar in magnitude.
Mammographic density is also associated with an increased risk of ER- and progesterone receptor–positive tumors, which suggests a hormonal etiology for mammographic density. A model that incorporates breast density calculates both the 5- and 10-year risk for breast cancer. Mammographic density may reflect high levels of circulating sex hormones or sensitivity to hormones but has a stronger predictive association with breast cancer than serum hormone levels alone. Indeed, endogenous sex hormone levels are strongly and independently related to the risk of breast cancer in postmenopausal women. Measurement of sex hormone levels has not gained wide acceptance, however, as a clinical risk assessment tool.
Clinical Risk Counseling
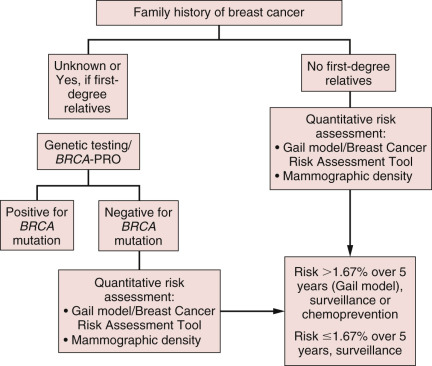
The major steps in risk assessment of breast cancer include (1) assessment of genetic susceptibility via genetic counseling and (2) quantitative risk assessment via the Gail model/Breast Cancer Risk Assessment tool and/or mammographic density analysis. These steps are outlined in Fig. 16.1 . Women at lower risk of breast cancer qualify for routine surveillance, including annual mammography for women older than 40 years of age as well as annual clinical breast examinations and self-breast examinations starting at 20 years of age. High-risk women who have a 5-year risk of breast cancer of 1.67% according to the Gail model/Breast Cancer Risk Assessment tool may qualify for chemoprevention in addition to routine or enhanced surveillance. Those options are discussed in the following sections.
Chemoprevention
Three areas unique to the field of chemoprevention must be considered in all stages of the clinical evaluation of a new chemopreventive agent. First, the characteristics of the target population must be clearly defined. For breast cancer chemoprevention, the target is a group of healthy women who may have had a previous diagnosis of breast cancer or who may be known to have a condition that predisposes them to the development of breast cancer. Second, the frequency and severity of side effects of the chemopreventive agent should be acceptable to the individual and ethically justifiable in the target population. Third, the duration of use of the chemopreventive agent must be defined. For most preventable malignancies, this requires a sustained period of drug administration that may be lifelong.
Epidemiologic studies indicate that estrogen-mediated events are integral to the development of breast cancer and support the hypothesis that intact ovarian function is required to develop breast cancer. Oophorectomy or radiation-induced ovarian ablation can reduce the incidence of breast cancer by up to 75%. These observations suggest that estrogen antagonists may be instrumental in the primary prevention of breast cancer.
Freedman and colleagues conducted a retrospective analysis that included data from the Study of Tamoxifen and Raloxifene (STAR) and Breast Cancer Prevention Trial (BCPT) trials described later in the chapter. They developed a benefit/risk index to quantify both beneficial and adverse outcomes from chemoprevention with the SERMs tamoxifen or raloxifene. This index helps decide whether to initiate chemoprevention by comparing the benefits and risks of raloxifene versus tamoxifen. Risks and benefits of treatment with raloxifene or tamoxifen depend on age, race, breast cancer risk, and history of hysterectomy. The benefits and risks of raloxifene and tamoxifen are described in tables ( Fig. 16.2 ) that can help identify groups of women for whom the benefits of chemoprevention outweigh the risks. The net benefit index is the expected number of life-threatening events (i.e., invasive breast cancer, hip fracture, endometrial cancer, stroke, and pulmonary embolism [PE]) and severe events (i.e., in situ breast cancer and deep vein thrombosis [DVT]) in 5 years with and without chemoprevention.
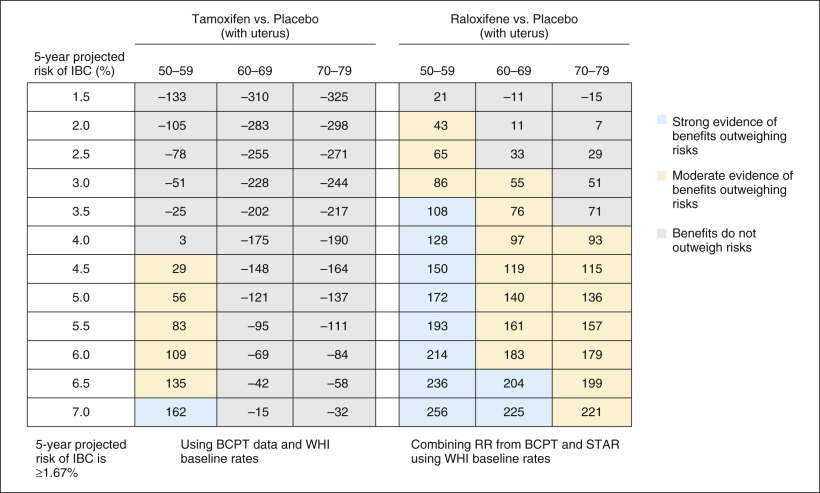
On the basis of these data, 9 million of the 65 million women aged 35 to 79 years in the United States with no history of breast cancer were eligible for tamoxifen chemoprevention based on inclusion criteria from the BCPT (described later in the chapter). Of these 9 million women, approximately 2.4 million would have derived a net benefit from taking tamoxifen based on their 5-year risk of developing breast cancer. An estimated 58,000 cases of invasive breast cancer would develop over the ensuing 5 years in that population. On the basis of the 49% risk reduction associated with tamoxifen in the BCPT, if all 2.4 million women had taken tamoxifen, more than 24,000 cases of breast cancer may have been prevented.
Tamoxifen
As already noted, hormones, especially estrogens, have been linked historically to breast cancer, with their role being attributed to their ability to stimulate cell proliferation. This cellular proliferation leads to the accumulation of random genetic errors that result in neoplastic transformation. According to this concept, chemoprevention of breast cancer is targeted to reduce the rate of cell proliferation through administration of hormonal modulators. Tamoxifen, a triphenylethylene compound, was synthesized in 1966 as a potential fertility agent. Several mechanisms have been proposed regarding tamoxifen’s ability to prevent or suppress breast carcinogenesis, including modulating the production of transforming growth factors (TGF-α and TGF-β) that regulate breast cancer cell proliferation; binding to cytoplasmic antiestrogenic binding sites, increasing intracellular drug levels; increasing sex hormone–binding globulin levels, which may decrease the availability of free estrogen for diffusion into tumor cells; increasing levels of natural killer cells; and decreasing circulating insulin-like growth factor-I levels, which may modify the hormonal regulation of breast cancer cell kinetics.
Raloxifene
Raloxifene hydrochloride, like tamoxifen, is a SERM that has antiestrogenic effects on breast and endometrial tissue and estrogenic effects on bone, lipid metabolism, and blood clotting. It is a benzothiophene with characteristics similar to but distinct from the triphenylethylene SERMs such as tamoxifen. In vivo studies demonstrated antitumor activity in carcinogen-induced tumors in rodents of a magnitude similar to that observed previously with tamoxifen. In vitro, raloxifene binds to both the alpha and beta subtypes of the ER (α and β, respectively).
Chemoprevention Risk-Reduction Trials
Four prospective studies evaluating tamoxifen for reducing the risk of invasive breast cancer have been published: the National Surgical Adjuvant Breast and Bowel Project (NSABP) Breast Cancer Prevention Trial (BCPT, P-1), the Royal Marsden Hospital (RMH) Tamoxifen Chemoprevention Trial, the Italian Tamoxifen Prevention Study, and the International Breast Intervention Study I (IBIS-I). A summary of the results of these trials is shown in Table 16.1 . Additional trials listed in the table evaluated raloxifene for the reduction of the risk of breast cancer in high-risk postmenopausal women. The findings from the largest of these studies in detail are reviewed in the following subsection. Data regarding arzoxifene and lasofoxifene are not reviewed because these SERMs have not been studied in large-scale, randomized, breast cancer risk-reduction clinical trials, and the agents are not approved by the FDA for this purpose.
| N | Recruitment Period | Treatment Groups and Daily Dose | Treatment Duration (Years) | Entry Criteria | Median Follow-Up (Months) | |
|---|---|---|---|---|---|---|
| NSABP BCPT | 13,205 | 1992–1997 | Placebo (6707) Tamoxifen 20 mg (6681) | 5 | >1%–6% 5-year risk | 57.6 |
| IBIS-I | 7,109 | 1992–2001 | Placebo (3566) Tamoxifen 20 mg (3573) | 5 | >2 times relative risk | 96.0 |
| Italian | 5,408 | 1992–1997 | Placebo (2708) Tamoxifen 20 mg (2700) | 5 | Normal risk, women with hysterectomy | 139.6 |
| Marsden | 2,471 | 1986–1996 | Placebo (1233) Tamoxifen 20 mg (1238) | 5-S | High risk, family history | 171.6 |
| MORE/CORE | 7,705/6,511 | 1994–1998/ 1998–2002 | Placebo (2576) Raloxifene 60 mg (2557)/ Placebo (2576) Raloxifene 120 mg (2572) | 4/8 | Normal risk, postmenopausal women with osteoporosis | 71.3 |
| RUTH | 10,101 | 1998–2000 | Placebo (5057) Raloxifene 60 mg (5044) | 5 | Normal risk, postmenopausal women with established or risk of coronary heart disease | 66.7 |
| STAR | 19,490 | 1999–2004 | Raloxifene 60 mg (9875) Tamoxifen 20 mg (9872) | 5 | >16% 5-year risk, postmenopausal women | 81.0 |
| GENERATIONS | 9,354 | 2004–2009 | Placebo (4678) Arzoxifene 20 mg (4676) | 4 | Normal-risk, postmenopausal with low bone density or osteoporosis | 54.3 |
| PEARL | 8,856 | 2001–2007 | Placebo (2852) Lasofoxifene 0–50 mg (2852) Lasofoxifene 0–25 mg (2852) | 5 | Normal-risk, postmenopausal women with osteoporosis | 59.6 |
Breast Cancer Prevention Trial
The BCPT, a randomized, placebo-controlled, double-blind clinical trial, was initiated in June 1992 by the collaboration of the NCI and the NSABP to evaluate whether tamoxifen reduced risk of invasive breast cancer in women at increased risk. It was the largest, prospective, controlled trial of tamoxifen’s risks and benefits in a high-risk population. The primary aim of the trial was to evaluate the effectiveness of 20 mg/day of tamoxifen orally for 5 years in preventing the occurrence of invasive breast cancer in women at high risk. Secondary aims of the trial were to assess osteoporotic fractures and cardiovascular disease in women taking tamoxifen compared with those in the control group.
Between June 1992 and September 1997, 13,388 women deemed at high risk for developing breast cancer were enrolled in the trial. Women were chosen if they were at high risk of developing breast cancer within the next 5 years if they met the following criteria: were 60 years of age or older, were between 35 and 59 years of age with a 5-year predicted risk of breast cancer of at least 1.66% as indicated by the Gail model, or had a history of LCIS. These women were then randomized to receive either 20 mg/day of tamoxifen ( n = 6681) or placebo ( n = 6707) for a period of 5 years.
The trial was terminated early when an interim analysis showed that statistical significance had occurred in a number of end points. This decrease was evident only in ER-positive breast cancers, with no significant change seen in ER-negative tumors. The median follow-up time at the end point was 48 months, at which time a 49% ( p < .00001) decreased risk of invasive breast cancer in the total study population was documented, with the greatest benefit seen in women 60 years of age and older. Overall, a total of 264 invasive cases were documented in a total of 13,175 women with measurable end points at the time of the interim analysis. Of the 264 cases, 175 cases occurred in the placebo group, compared with 89 cases in the tamoxifen group, a 49% reduction in the incidence of invasive breast cancer.
Other Outcomes in the Breast Cancer Prevention Trial
Secondary outcomes in the BCPT included osteoporotic fractures and cardiovascular events. Tamoxifen is known to have estrogen agonist–like effects on both mineral density and serum cholesterol levels in postmenopausal women. During the BCPT, women in the tamoxifen group had a 19% reduction in hip, spine, and distal radius fractures. Thirty-four of the women enrolled in the trial experienced a hip fracture; when the tamoxifen versus the placebo group were compared, there was a 45% reduction, which failed to reach statistical significance because of the small number of events that occurred. The incidence of cardiovascular events (namely, stroke and transient ischemic attack) revealed no statistically significant difference between the tamoxifen and placebo group likely because only 30% of women in the trial were aged 60 years or older.
Few clinically significant differences in quality-of-life outcomes were seen when comparing the tamoxifen and placebo groups, and tamoxifen was not associated with an increased risk of developing depressive symptoms in the BCPT.
Other Unfavorable Outcomes in the Breast Cancer Prevention Trial
Adverse outcomes related to tamoxifen in the BCPT included PE, DVT, endometrial carcinoma, cataracts, and vasomotor symptoms. These outcomes were significantly higher in women older than 50 years of age compared with their younger counterparts. Women on tamoxifen were found to have a statistically significant higher incidence of PE than those on placebo. Although incidences of DVT, stroke, and transient ischemic attack in these women were not statistically significant, incidence in women on tamoxifen was higher.
Women in the tamoxifen arm of the trial were found to have a 2.5 times greater risk of developing invasive endometrial carcinoma than women in the placebo arm, with an annual incidence of 2.3 per 1000 in the tamoxifen arm and 0.9 per 1000 in the placebo arm. This increased risk was greater in postmenopausal women. All cases of endometrial carcinoma that occurred in the BCPT were International Federation of Gynecology and Obstetrics (FIGO) stages 0 or I and had excellent clinical prognoses with treatment. A marginal increase of 14% in the development of cataracts was seen in women who were free of cataracts at initiation of the trial. The number of cataract surgeries was also increased in women taking tamoxifen. Vasomotor symptoms, mainly hot flashes, were reported by 46% of women on tamoxifen and only 29% of women in the placebo arm, whereas an increase in vaginal discharge was reported in 29% of women taking tamoxifen and 13% of women taking placebo.
After 7 years of follow-up in the BCPT, the cumulative rate of invasive breast cancer was reduced from 42.5 per 1000 in the placebo group to 24.8 per 1000 in the tamoxifen group, a 43% reduction in risk, and the cumulative rate of noninvasive breast cancer was reduced from 15.8 per 1000 in the placebo group to 10.2 per 1000 in the tamoxifen group (37% reduction) ( Fig. 16.3 ). Tamoxifen continued to reduce the occurrence of ER-positive tumors by 69%, but no difference was seen in the occurrence of ER-negative tumors. Also after 7 years, risks of PE were approximately 11% lower than in the original report, and risks of endometrial cancer were about 29% higher, but these differences were not statistically significant. The net benefit achieved with tamoxifen varied according to age, race, and level of breast cancer risk. Despite the potential bias caused by the unblinding of the P-1 trial and subsequent crossover between the treatment groups, the magnitudes of all beneficial and undesirable treatment effects of tamoxifen were similar to those initially reported, with notable reductions in breast cancer and increased risks of thromboembolic events and endometrial cancer. The incidence of all osteoporotic fractures was reduced by 19% in women taking tamoxifen compared with those in the placebo group. There was a 45% reduction in fractures of the hip that missed reaching statistical significance because of the small number of events reported.
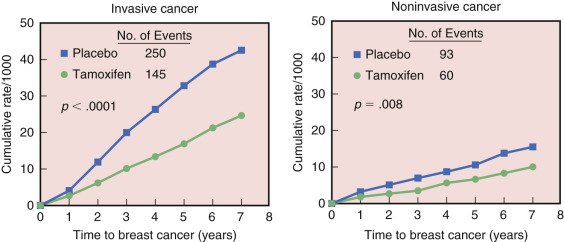
In summary, the BCPT found that tamoxifen greatly reduced the incidence of ER-positive invasive and noninvasive breast cancers compared with placebo over the 84-month follow-up time.
International Breast Cancer Intervention Study I
The IBIS-I trial, a randomized, placebo-controlled study, with design and outcomes similar to that of BCPT, was initiated to evaluate whether tamoxifen reduced the risk of invasive breast cancer in women at increased risk. The primary aim of the trial was to evaluate the effectiveness of 20 mg/day of tamoxifen given for 5 years in preventing the occurrence of both invasive and in situ breast cancer in women deemed at high risk compared with placebo.
More than 7000 women 35 to 70 years of age evaluated as high risk for development of breast cancer (invasive or in situ) were enrolled into the trial and randomly assigned to either the tamoxifen or placebo for 5 years. Selection criteria for the trial’s high-risk patients required that women 45 to 70 years of age have at least a twofold relative risk, women 40 to 44 years of age at least a fourfold relative risk, and women 35 to 39 years of age at least a tenfold relative risk of developing breast cancer. Risk factors involved in determining the relative risk of breast cancer development included family history, history of LCIS, history of atypical hyperplasia, benign breast biopsies, and nulliparity. Unlike the BCPT, and like the Italian trial discussed subsequently, women in this trial were permitted use of postmenopausal hormone replacement therapy (HRT), with approximately 40% of women using HRT at some time during the trial. On the basis of the published model, women in this trial were at moderately increased risk of development of breast cancer ( Fig. 16.4 ).
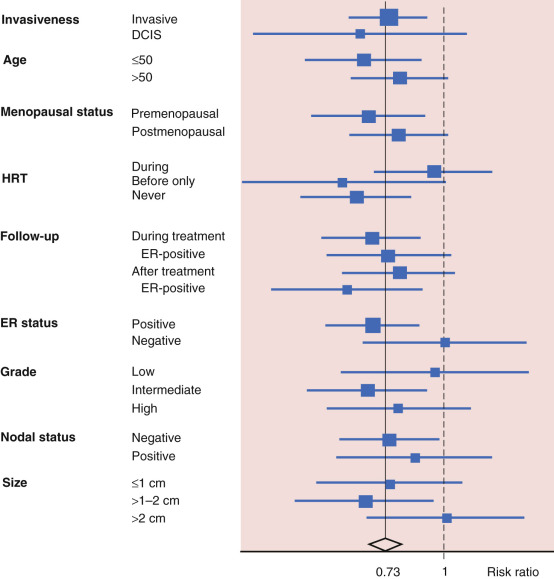
Analysis with a median follow-up of 96 months after randomization in IBIS-I revealed a total of 337 cases of breast cancer that had been diagnosed, 142 breast cancers were diagnosed in the tamoxifen group and 195 in the placebo group, a 42% reduction in risk. The risk-reducing effect of tamoxifen was constant for the entire follow-up period, and no lessening of benefit was observed for up to 10 years after randomization. There was a statistically insignificant interaction between HRT use and treatment with tamoxifen in women in IBIS-I. In women who never used HRT or who used it only before the trial, there was a statistically significant reduction in ER-positive breast cancers in the tamoxifen group compared with the placebo group (a 38% reduction for all breast cancers). For women who used HRT during any point of the trial, no clear benefit of tamoxifen was seen in reducing the risk of breast cancer, either overall (66 vs. 69 cases, relative risk [RR] = 0.92, 95% confidence interval [CI] = 0–1.31) or for ER-positive tumors (40 vs. 43 cases, RR = 0.89, 95% CI = 0.57–1.41). Results were similar regardless of the HRT preparations used (i.e., either estrogen only or combined estrogen and progestin). HRT use was not associated with the development of ER-negative breast cancers, either during the active treatment period or during subsequent follow-up. The risk reduction observed may be smaller that that seen in the BCPT both because patients enrolled onto IBIS-I were allowed to take HRT during the trial and because few women in IBIS-I had atypical hyperplasia, whereas a large reduction in incidence of invasive breast cancer was seen in BCPT.
As in the BCPT trial, adverse outcomes in the tamoxifen arm in the IBIS-I included an increase in thromboembolic events, a marginal increase in risk of endometrial cancer, and an overall increase in risk of death from all causes. The overall risk of clotting events was increased in tamoxifen users, with a 2.5-fold increase in risk of venous thromboembolism. As with the BCPT, this risk was seen predominately in women older than 50 years of age and in those women with a recent history of surgery. There was a marginal, nonstatistically significant increase in the risk of endometrial cancer in women taking tamoxifen, especially in women aged 50 years and older. As observed in the BCPT, all cases of endometrial cancer diagnosed were FIGO stages 0 or I.
Active treatment was discontinued after 5 years. After a median follow-up that extended to 16 years, breast cancers were diagnosed 7.0% of the women in the tamoxifen group versus 9.8% of 3575 women in the placebo group of IBIS-I, an enduring 30% reduction in the risk of all breast cancer. The risk of developing breast cancer was similar between years 0 to 10 and after 10 years. The greatest reduction in risk was seen in invasive ER-positive breast cancer (34%) and DCIS (35%), but no effect was noted for invasive ER-negative breast cancer. These results indicate that tamoxifen offers a long period of protection after treatment cessation and substantially improves the benefit-to-harm ratio of the drug for breast cancer prevention.
Summary of the SERM Chemoprevention Trials
Additional clinical trials that had experimental designs similar to those trials reviewed earlier are summarized in Table 16.2 . It should be noted that in the Italian Tamoxifen Prevention Trial, women were eligible for the trial if they were between ages 35 and 70 years and had undergone hysterectomy for benign disease because of the associated risk of endometrial cancer in patients taking tamoxifen. Women in the trial were not required to undergo standard breast cancer risk assessment. Thus some of the participants in the trial were at decreased risk for developing breast cancer at randomization because they had had previous oophorectomy (48.3%) before menopause. Concomitant use of HRT was also permitted. Accrual to the trial was ended prematurely because of a 26.3% dropout rate for women already randomized secondary to side effects, decreased interest, and fear.
| Overall (Invasive and DCIS) | Annual Rates Per 1000 in Control Group | HR (95% CI) (Tamoxifen vs. Placebo) | ER-Positive Invasive | HR (95% CI) | ER-Negative Invasive | HR (95% CI) | DCIS | HR (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|
| Tamoxifen Trials | |||||||||
| Marsden | 96 vs. 114 | 6.4 | 0.87 (0.63–1.21) | 51 vs. 83 | 0.66 (0.44–0.99) | 25 vs. 17 | 1.66 (0.81–3.40) | 14 vs. 9 | 1.40 (0.44–4.40) |
| IBIS-I | 143 vs. 198 | 6.7 | 0.72 (0.58–0.90) | 88 vs. 131 | 0.69 (0.52–0.90) | 36 vs. 38 | 0.97 (0.62–1.54) | 16 vs. 27 | 0.52 (0.27–0.99) |
| NSABP-P-1 | 130 vs. 248 | 6.1 | 0.52 (0.42–0.64) | 44 vs. 134 | 0.33 (0.23–0.46) | 39 vs. 31 | 1.26 (0.78–2.02) | 38 vs. 70 | 0.54 (0.36–0.80) |
| Italian | 62 vs. 74 | 4.2 | 0.83 (0.58–1.19) | 36 vs. 48 | 0.73 (0.45–1.17) | 16 vs. 17 | 0.87 (0.43–1.79) | 9 vs. 6 | 1.80 (0.60–5.38) |
| Total (0–10 years) | 431 vs. 634 | 0.67 (0.59–0.76) | 219 vs. 396 | 0.56 (0.47–0.67) | 116 vs. 103 | 1.13 (0.86–1.49) | 77 vs. 112 | 0.72 (0.57–0.92) | |
| Total (0–5 years) | 256 vs. 409 | 0.62 (0.53–0.73) | 121 vs. 235 | 0.51 (0.41–0.64) | 78 vs. 76 | 1.03 (0.75–1.41) | 47 vs. 83 | ||
| Total (5–10 years) | 175 vs. 225 | 0.78 (0.62–0.97) | 98 vs. 161 | 0.63 (0.47–0.83) | 38 vs. 27 | 1.55 (0.88–2.72) | 30 vs. 29 | ||
| Raloxifene Trials | |||||||||
| MORE/CORE | 57 vs. 65 | 4.2 | 0.42 (0.29–0.60) | 22 vs. 44 | 0.24 (0.15–0.40) | 15 vs. 7 | 1.06 (0.43–2.59) | 13 vs. 7 | 0.91 (0.36–2.28) |
| RUTH | 52 vs. 76 | 4.2 | 0.67 (0.47–0.96) | 25 vs. 55 | 0.45 (0.28–0.72) | 13 vs. 9 | 1.44 (0.61–3.63) | 11 vs. 5 2 | 17 (0.75–6.25) |
| STAR (tamoxifen vs. raloxifene) | 358 vs. 447 | 5.9 | 0.81 (0.70–0.93) | 182 vs. 221 | 0.83 (0.69–1.02) | 60 vs. 70 | 0.79 (0.56–1.11) | 111 vs. 137 | 0.82 (0.64–1.05) |
| Total (0–10 years) | 467 vs. 588 | 0.66 (0.55–0.80) | 229 vs. 320 | 0.44 (0.34–0.58) | 88 vs. 93 | 1.37 (0.96–1.95) | 135 vs. 149 | 1.07 (0.68–1.68) | |
| Total (0–5 years) | 327 vs. 421 | 0.63 (0.51–0.79) | 168 vs. 224 | 0.40 (0.29–0.56) | 62 vs. 71 | 1.27 (0.83–1.95) | 86 vs. 108 | 1.08 (0.60–1.96) | |
| Total (5–10 years) | 140 vs. 167 | 0.84 (0.51–1.27) | 61 vs. 96 | 0.72 (0.49–1.06) | 26 vs. 22 | 1.70 (0.84–3.47) | 49 vs. 41 | 0.88 (0.45–1.74) | |
| Pearl a | |||||||||
| 0.25 mg | 20 vs. 24 | 2.0 | 0.82 (0.45–1.49) | 9 vs. 18 | 0.49 (0.22–1.10) | 7 vs. 2 2 | 2.83 (0.57–14.02) | 4 vs. 4 | 0.99 (0.25–3.99) |
| 0.5 mg | 5 vs. 24 | 2.0 | 0.21 (0.08–0.55) | 3 vs. 18 | 0.17 (0.05–0.56) | 0 vs. 2 | 3 vs. 4 | 0.50 (0.09–2.73) | |
| All Trials b | |||||||||
| Overall | Annual Rates Per 1000 | HR (95% CI) | ER-Positive Invasive | HR (95% CI) | ER-Negative Invasive | HR (95% CI) | DCIS | HR (95% CI) | |
| Total (0–10 years) | 587 vs. 852 | 4.7 | 0.62 (0.56–0.69) | 287 vs. 543 | 0.49 (0.42–0.57) | 160 vs. 131 | 1.14 (0.90–1.45) | 110 vs. 138 | 0.69 (0.53–0.90) |
| 0.61 (0.49–0.75) | 0.44 (0.33–0.60) | 1.14 (0.90–1.45) | 0.79 (0.53–1.19) | ||||||
| Total (0–5 years) | 376 vs. 594 | 4.6 | 0.58 (0.51–0.66) | 174 vs. 360 | 0.45 (0.38–0.54) | 111 vs. 90 | 1.05 (0.80–1.39) | 73 vs. 107 | 0.66 (0.48–0.90) |
| 0.58 (0.47–0.73) | 0.42 (0.29–0.61) | 1.05 (0.80–1.39) | 0.73 (0.47–1.14) | ||||||
| Total (5–10 years) | 211 vs. 258 | 4.9 | 0.75 (0.61–0.93) | 113 vs. 183 | 0.58 (0.45–0.76) | 49 vs. 32 | 1.66 (0.98–2.81) | 37 vs. 31 | 0.94 (0.53–1.66) |
| 0.75 (0.59–0.94) | 0.58 (0.45–0.76) | 1.66 (0.98–2.81) | 0.94 (0.53–1.66) | ||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree