Primary neoplasms of the liver
Junichi Shindoh, MD, PhD  Kristoffer W. Brudvik, MD, PhD
Kristoffer W. Brudvik, MD, PhD  Jean-Nicolas Vauthey, MD
Jean-Nicolas Vauthey, MD
Overview
Primary liver cancer is the second leading cause of cancer death in men and the sixth leading cause among women. Hepatocellular carcinoma (HCC) constitutes 70–85% of primary hepatic neoplasms, followed by intrahepatic cholangiocarcinoma (ICC) (10–15%) and other less common hepatic malignancies (5%) such as hepatic angiosarcoma, epithelioid hemangioendothelioma, or hepatic lymphoma. Primary hepatic malignancies are generally associated with poor survival, and only a limited evidence of systemic therapy have been available, prevention, surveillance, early diagnosis, and multidisciplinary treatment approach are important factors to maximize treatment outcomes. In addition, most of the primary hepatic neoplasms are associated with chronic liver disease or cirrhosis, and the decreased hepatic functional reserve often precludes aggressive treatment for tumors. Therefore, for treatment selection, one should consider two intrinsic conflicting factors, curability and safety of treatment. Currently, several clinical staging systems and treatment algorithms are available to adequately select therapeutic options for HCC. The choice of therapy is individualized based on the tumor burden, degree of underlying liver disease, patient performance status, and the overall possibility of side effects or complications balanced with acceptable results. Selection of treatment should be determined in a multidisciplinary approach with the local expertise. The most curative treatment options including surgical resection, radiofrequency ablation, and orthotopic liver transplantation (OLT) should be the first priority as long as such treatments are approved. For the other hepatic malignancies, only limited clinical evidence is available and surgical resection or OLT is considered as the first-line treatment.
The liver is a specific site where all the visceral venous flow passes through before it reaches the heart. Therefore, the liver is the most common site of metastases from gastrointestinal malignancies which is termed secondary hepatic neoplasms. However, tumors can also arise from the cells within the liver and are termed primary hepatic neoplasms. Of the primary hepatic neoplasms, hepatocellular carcinoma (HCC) is the most common malignancy constituting 70–85% of the primary liver cancer, followed by intrahepatic cholangiocarcinoma (ICC) (10–15%) and other less common hepatic malignancies (5%) such as hepatic angiosarcoma, epithelioid hemangioendothelioma (EHE) or hemangiopericytoma, or hepatic lymphoma. Because primary hepatic malignancies are generally associated with poor survival and only a limited evidence of systemic therapy have been available, early diagnosis and multidisciplinary treatment approach are of most importance to expect favorable long-term outcomes. This chapter will review and discuss the clinical features and current treatment approaches for HCC, ICC, and the other less common primary liver cancers.
Hepatocellular carcinoma
Incidence and epidemiology
Primary liver cancer is the fifth most common cancer in men and the seventh in women, and the second leading cause of cancer death in men and the sixth leading cause among women, with about 695,500 deaths annually worldwide.1 Nearly 85% of the cases occur in developing countries especially in sub-Saharan Africa and in East and Southeast Asia, with typical incidence rates of >20 per 100,000 individuals. The incidence rates are generally lowest in developed countries, with the exception of Japan where hepatitis C virus (HCV) infection is the most common cause for HCC.
Globally, the incidence of HCC is increasing in areas with historically low rates, including parts of Oceania, Central Europe, and North America.2, 3 A study reported in 2009 indicated that age-adjusted incidence rates of HCC tripled between 1975 and 2005 in the United States from 1.6 to 4.9 per 100,000 people.2 In contrast, the incidences of liver cancer are decreasing in historically high-risk areas, such as China and Singapore, most likely due to reduction in hepatitis B virus (HBV) infection through improved public health.
The rates of HCC are more than twice as high in men as in women across geographic regions.4 The incidence of HCC increases with age, although the age threshold varies among countries because of differences in predominant etiologies for HCC. The age threshold for HCC is generally younger when the predominant risk factor is vertical transmission of HBV (e.g., Southeast Asia and Africa), and older where acquired HCV infection during adulthood is the most common cause of HCC (e.g., Japan and United States).
Risk factors
Cirrhosis is the main risk factor and present in 80–90% of patients with HCC. It is hypothesized that chronic hepatocellular injury and inflammation from a variety of causes lead to cirrhosis and HCC as a result of hepatocyte regeneration and hyperplasia predisposing to mutations and malignant transformation.
Viral hepatitis (HBV and HCV)
It has been estimated that 75–80% of HCC are associated with chronic infections with HBV (50–55%) or HCV (25–30%).5 Transmission of HBV occurs during delivery (vertical transmission), blood transfusions, sexual intercourse, or intravenous drug abuse. Transmission of HCV can also occur via parenteral exposure, however, primarily through blood transfusions and intravenous drug abuse.
HBV is a double-stranded DNA virus and chronic infection to HBV is the most common etiology for HCC, especially in China and Korea. Patients with positive hepatitis B surface antigen (HBsAg) are at high risk of developing cirrhosis and HCC. However, it has been reported that those who are positive for anti-hepatitis B core antibodies (HBcAb) are also at high risk for HCC even when HBs-Ag is serologically negative.6 In contrast to HCV-related HCC which usually arises in the liver with severe fibrosis or cirrhosis, approximately 20% of HBV-related HCC occurs in the absence of cirrhotic changes. A direct mutagenic effect that causes carcinogenesis has been postulated for HBV. Integration of HBV DNA in the host genome adjacent to cellular oncogenes or transactivating effect of the HBx protein on cellular gene expression may be attributable to the development of HCC without cirrhosis.7–9 In fact, HBV-DNA integration has been detected in hepatocytes before tumor development among patients positive for HBsAg, which may enhance chromosomal instability and facilitate HCC development.10, 11
HCV is a small, single-stranded RNA virus. The prevalence of HCV infection varies widely according to geographical areas. A meta-analysis of 21 case–control studies reported that HCC risk was 17 times higher among HCV-positive individuals as compared to HCV-negative individuals.12 It has been suggested that oxidative stress is one of the mechanisms involved in inflammation-related carcinogenesis in patients infected with HCV.13 In response to viral antigens, the activated macrophages and other recruited leukocytes release reactive oxygen species, causing areas of focal necrosis and compensatory cell division.14 When these oxidants overwhelm the antioxidant defenses of neighboring cells, damages to biomolecules, particularly to oncogenes or tumor suppressor genes, may increase the carcinogenic potential of the underlying liver. HCV displays a high genetic variability. On the basis of nucleotide sequence homology, whole-sequenced HCV isolates are classified as genotype 1a, 1b, 2a, and 2b. The geographic distribution of these genotypes demonstrated that genotypes 1a, 1b, and 2a are predominant in Western countries and East Asia, whereas genotype 2b is predominant in the Middle East.15 The HCV genotype 1b is reportedly more aggressive and more closely associated with advanced chronic liver diseases such as liver cirrhosis and HCC. However, these observations can be partially explained by the refractoriness to antiviral therapy, and recent studies have reported that lower HCV viral load is correlated with improved survival outcomes after surgical resection of HCC irrespective of the genotypes of HCV.16, 17
Alcohol
Excessive alcohol consumption is a major risk factor for chronic liver disease and HCC, causing various degrees of liver damage from simple fat accumulation to cirrhosis. Various studies have concluded that excessive alcohol intake is an important risk factor for HCC development. A US case–control study demonstrated approximately threefold increase in HCC risk among individuals with heavy alcohol consumption defined as more than 60 ml ethanol per day.18 HCC rarely develops in the absence of cirrhosis; however, the risk is increased with concurrent HBV or HCV infection.19, 20 A meta-analysis of 20 studies published between 1995 and 2004 that involved more than 15,000 patients with chronic HCV infection reported that the pooled relative risk of cirrhosis associated with heavy alcohol intake was 2.33, compared with no or low-quantity alcohol intake.19 Therefore, alcohol consumption should be avoided among patients with viral hepatitis.
Aflatoxins
Aflatoxins (AFs) are potent hepatocarcinogens produced by Aspergillus flavus and Aspergillus parasiticus that grow readily on foods such as corn and peanuts stored in warm, damp conditions. There are four AF compounds: B1, B2, G1, and G2, and the most common and most toxic AF compound is AFB1. When ingested, AFB1 is metabolized to a highly active 8,9-epoxide metabolite, which can bind to and damage DNA. A consistent genetic mutation in codon 249 in the tumor suppressor p53 gene has been identified and positively correlated with AF exposure.21, 22 Although AFB1 contributes to hepatocarcinogenesis, its role in pathogenesis of HCC is primarily mediated by its synergic effects on chronic hepatitis B because the areas where AFB1 exposure is an environmental problem also have a high prevalence of chronic HBV infection. A prospective study from China showed that urinary excretion of AF metabolites was associated with increased risk for HCC up to fourfold, and HBV infection independently increased the risk sevenfold. However, the patients who excreted AFB1 metabolites and had concomitant infection of HBV showed a 60-fold increase in risk of development of HCC.23 These results suggest that prevention of HBV-related HCC would reduce the effects of AF on HCC risk.
Obesity
It is well established that obesity is significantly associated with a wide spectrum of hepatobiliary diseases, including fatty liver diseases, steatosis, steatohepatitis, and cryptogenic cirrhosis.24, 25 In a large prospective cohort study of more than 900,000 individuals in the United States followed up for a 16-year period, liver cancer mortality rates were five times greater among men with the greatest baseline body mass index (range, 35–40) compared with those with a normal body mass index, whereas the risk of liver cancer was not as increased in women, with a relative risk of 1.68.26 Two other population-based studies from Northern Europe have also shown that obesity is correlated with increased HCC risk.27, 28
Diabetes mellitus
Recently, diabetes has been recognized as a risk factor for chronic liver disease and HCC. Although adjustment of potential biases in cross-sectional and case–control studies is difficult, several studies have clearly demonstrated positive correlation between diabetes and HCC.29–33 The postulated mechanisms for liver cell damage induced by type 2 diabetes mellitus involve insulin resistance and hyperinsulinemia.34, 35 Hyperinsulinemia reportedly reduces liver synthesis and blood levels of insulin growth factor-binding protein-1 (IGFBP-1), which increases (1) bioavailability of insulin-like growth factor-1 (IGF-1), (2) promotion of cellular proliferation, and (3) inhibition of apoptosis.36 Excessive insulin binds to insulin receptor and activates its intrinsic tyrosine kinase, leading to phosphorylation of insulin receptor substrate-1 (IRS-1),37 and hyperinsulinemia is also associated with production of reactive oxygen species, which may cause damage to DNA. Overexpression of IRS-1 is associated with decreased apoptosis, which is mediated by transforming growth factor β.38 Given these evidences in basic researches and pathologic observation that HCC tumor cells overexpress both IGF-1 and IRS-1,39 diabetes mellitus seems to contribute to liver cell damage and development of HCC.
Hemochromatosis
Hemochromatosis is an autosomal recessive genetic disorder of iron metabolism, with a prevalence rate of 2–5 per 1000 in the Caucasian population. In this inherited disorder, mutations have been documented in the HFE gene on chromosome 6 (Cys282Tyr [C282Y] and His63Asp [H63D]), the HFR-2 gene on chromosome 1, and the HFE-3 gene on chromosome 7. The C282Y mutation is the most commonly detected mutation and C282Y homozygotes or C282Y/H63D compound heterozygotes40–42 is associated with increased absorption of dietary iron and accumulation in tissues such as the skin, heart, and liver, resulting in heart failure or liver cirrhosis.
There is growing evidence that even mild accumulation of iron is harmful to the liver, especially when other hepatotoxic factors such as chronic viral hepatitis or heavy alcohol intake are present. Iron enhances the pathogenicity of microorganisms, adversely affects the function of macrophages and lymphocytes, and enhances fibrogenic pathways.43 A synergistic relationship between HCV infection and iron overload has been suggested,44 and iron depletion has been reported to improve liver function tests in patients with chronic hepatitis C.45 Although diagnosis of hemochromatosis is relatively difficult, treatment with therapeutic phlebotomy or iron chelation therapy before onset of cirrhosis may be effective in preventing the development of cirrhosis and HCC in these patients.
α1-Antitrypsin deficiency
α1-Antitrypsin deficiency (AATD) is an autosomal dominant disorder with mutations in the serine protease inhibitor (Pi) gene. Over 75 different Pi alleles have been identified, most of which are not associated with the disease,46 and a relationship exists between Pi phenotypes and serum concentrations of α1-antitrypsin. Typical clinical presentation of patients with AATD includes emphysema, hepatic necroinflammation, cirrhosis, and HCC. Because no effective medical treatment is available, liver transplantation is indicated for patients with decompensated cirrhosis in correcting the underlying metabolic disorder.
Other potential risk factors
The higher incidence of HCC reported in men compared to women suggest the influence of hormonal factors on hepatocarcinogenesis. Long-term use of oral contraceptives has been thought to be a potential risk factors for HCC; however, a review of 12 case–control studies have yielded an overall adjusted odds ratio of 1.6 (95% CI, 0.9–2.5),47 and the correlation between the oral contraceptives and risk for HCC remains inconclusive.
Hypothyroidism has also been reported to be a potential risk factor for HCC especially in women.48, 49 Patients with hypothyroidism may experience weight gain50 and insulin resistance,51, 52 both of which are significant factors for nonalcoholic steatohepatitis. Although the actual mechanism is unclear and only limited clinical evidence is available, development of chronic liver disease and hepatocarcinogenesis might be influenced by these hormonal factors to some extent.
Prevention
Prevention of HCC is highly dependent on avoiding the risk factors, adequate treatment for the underlying chronic liver disease, and early diagnosis of precursor lesions. Because the most common cause of HCC is cirrhosis associated with viral hepatitis, prevention of infection to HBV/HCV and treatment of chronic hepatitis with antiviral therapy are of most importance in cancer prevention.
For hepatitis B, parenteral exposure is the most common cause of transmission in developing countries and improvements in hygiene conditions and public education are essential to avoid viral transmission. In addition, vaccination and effective antiviral therapy are currently available for HBV. After the initiation of HBV vaccination, significant declines in the incidence of HCC have been documented in high-risk countries such as Taiwan.53 When HBV infection has been established, various effective antiviral agents are currently available and reduction of viral load has reportedly correlated with reduced risk of HCC and improved survival outcomes in patients with chronic hepatitis B.54–56
For hepatitis C, interferon (IFN)-based combination treatment has been the standard of care and reduction of HCC risk has been shown especially in patients who achieved sustained viral response (SVR).57–60 However, genetic variability of HCV highly affects the effectiveness of antiviral therapies. Although only a few clinical evidences are available, recently introduced new direct-acting antiviral agents (DAAs) have dramatically improved virologic response rate and will likely contribute to prevent HCC among patients with chronic hepatitis C in the near future.61–63
Pathology
The malignant transformation of hepatocytes to HCC is considered as a multistep process associated with genetic mutations, allelic losses, epigenetic alterations, and perturbation of molecular cellular pathways. However, the molecular process of carcinogenesis in HCC is poorly understood. The phenotypic expression of these potential multistep changes can be manifested by precursor lesions that can be distinguishable from surrounding regenerative nodules associated with cirrhosis with regard to size, color, texture, and degree of bulging of the cut surface. Changes in the tumor blood supply correspond to each step of the multistep process, from precursor lesions to classical HCC, and helps differential diagnosis on dynamic studies in CT or MRI (Figure 1).
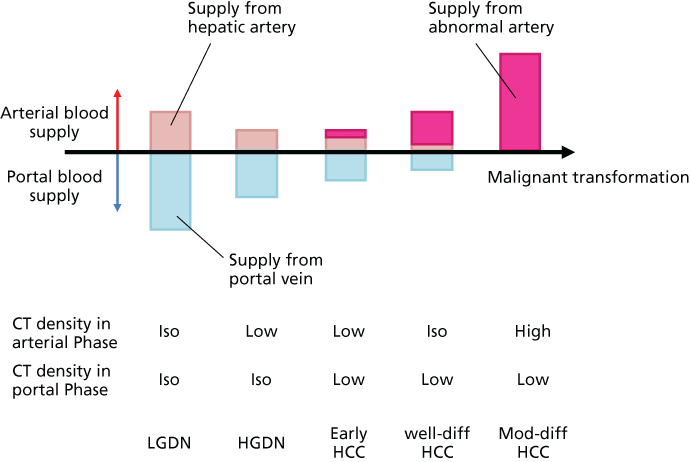
Figure 1 A model of dynamic changes in blood supply from precursor lesions to classical hepatocellular carcinoma. Dynamic changes in arterial and portal supply and exponential increase in abnormal arterial supply correlate with the typical patterns of enhancement in dynamic CT or MRI. Abbreviations: LGDN, low-grade dysplastic nodule; HGDN, high-grade dysplastic nodule; HCC, hepatocellular carcinoma; well-diff, well differentiated; mod-diff, moderately differentiated.
Dysplastic nodules
It is commonly accepted that there is a stepwise progression from cirrhotic nodule to HCC. A unified nomenclature of such liver nodules has recently been reviewed by the International Working Party of the World Congress of Gastroenterology.64 Dysplastic nodule (DN) is a distinct nodular lesion >5 mm in diameter and subclassified into low-grade dysplastic nodule (LGDN) and high-grade dysplastic nodule (HGDN). LGDNs show only mild dysplasia without architectural atypia, whereas HGDNs are characterized by architectural and/or cytologic atypia, which is insufficient for a diagnosis of HCC. HGDNs often show increased cell density with an irregular trabecular pattern. Small cell change (small cell dysplasia) is the most frequently seen form of cytologic atypia in HGDNs.
Early HCC
Early HCCs are vaguely nodular up to around 2 cm in diameter and are characterized by various combinations of the following major histologic features.64
- 1. Increased cell density more than two times that of the surrounding tissue, with an increased nuclear/cytoplasm ratio and irregular thin-trabecular pattern.
- 2. Varying numbers of portal tracts within the nodule (intratumoral portal tracts).
- 3. Pseudoglandular pattern.
- 4. Diffuse fatty change.
- 5. Varying numbers of unpaired arteries.
Distinguishing early HCCs from HGDNs is an unresolved challenge. Stromal invasion, defined as the presence of tumor cells invading into the portal tracts or fibrous septa, has been proposed as the most relevant feature discerning early HCC from HGDN. However, such feature may be difficult to identify, especially on biopsy specimens. In that context, a panel of three immunohistochemical markers of malignant transformation, heat shock protein 70, glutamine synthetase, and glypican 3, has been used to distinguish HCCs from HGDNs as well as CK 7 immunostaining for recognition of stromal invasion in early HCC.65, 66
Macroscopic presentation of HCC
HCC forms a soft mass with a heterogeneous macroscopic appearance, polychrome with foci of hemorrhage or necrosis (Figure 2). Grossly, three main growth patterns that were described by Eggel in 1901 remain widely used.67 The nodular type consists of well-circumscribed tumor nodules. Massive HCCs are circumscribed, huge tumor masses occupying most or all of a hepatic lobe. This type is commonly observed in patients without cirrhosis. The diffuse type is rare and characterized by innumerable indistinct small nodules studding the entire liver. The different patterns of growth are associated with various risks of spread, both intrahepatic and extrahepatic.68 The Liver Cancer Study Group of Japan (LCSGJ) has proposed a modification, with the nodular category being divided into three subtypes: single nodular subtype, single nodular subtype with perinodular tumor growth, and confluent multinodular subtype.69
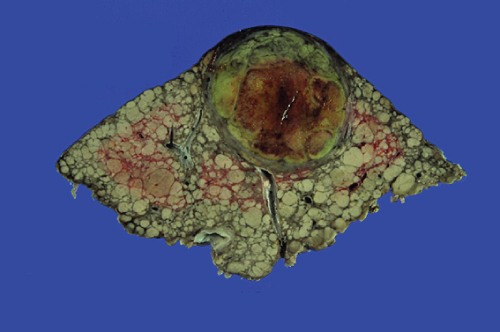
Figure 2 Gross appearance of hepatocellular carcinoma.
Multicentricity of tumor is noted in 16–74% of HCCs resected in cirrhotic livers.68–72 Although it is sometimes difficult to distinguish intrahepatic metastases from HCCs generated by multicentric neocarcinogenesis, tumor nodules are considered as intrahepatic metastases when (1) they show a portal vein tumor thrombus or grow contiguously with a thrombus, (2) multiple small satellite nodules surrounding a larger main tumor, or (3) a single lesion is adjacent to the main tumor but is significantly smaller in size and presents the same histology.73
Vascular invasion and formation of tumor thrombus are frequently observed especially in poorly differentiated HCC or large tumor. The portal vein is the most common site of vascular invasion, followed by hepatic veins, biliary tract, and hepatic arteries. The degree of tumor extension through the vascular structure is closely associated with prognosis. When a portal vein tumor thrombus extends up to the left or right portal pedicle (Vp3) or main portal trunk (Vp4), most patients develop recurrence and die within 2 years after surgery.74
Microscopic presentation of HCC
Grading of HCC has relied on Edmondson and Steiner classification for many years, which divided HCC into four grades from I to IV on the basis of histological differentiation.75 Tumors with well-differentiated neoplastic hepatocytes arranged in thin trabeculae correspond to grade I. The larger and more atypical neoplastic cells are sometimes organized in an acinar pattern in grade II. Architectural and cytologic anaplasia are prominent in grade III, but the neoplastic cells are readily identified as hepatocytic in origin. When composed of markedly anaplastic neoplastic cells not readily identified as hepatocytic origin, the tumor is classified as grade IV.
Histologic variants of HCC
Fibrolamellar HCC
Fibrolamellar variant is a rare entity accounting for <1% of all cases of primary liver cancer, which is commonly encountered in young population without chronic liver disease. Fibrolamellar carcinomas are firm, sharply demarcated, and usually single tumors, ranging from 5 cm to over 20 cm. Histologically, it is characterized by the presence of lamellar stromal bands surrounding nests of large polygonal eosinophilic tumor cells with prominent nucleoli. Intensive surgical approaches have been reported to offer a chance of long-term survival despite occurrence of extrahepatic recurrences for patients with fibrolamellar HCC.76
Combined HCC and cholangiocarcinoma
Combined HCC and cholangiocarcinoma (HCC-CC) contain unequivocal elements of both HCC and ICC. These tumors show variable combinations of characteristic features of HCC (e.g., bile production, intercellular bile canaliculi, or a trabecular growth) as well as ICC (e.g., glandular structures, intracellular mucin production, or immunoreactivity for MUC-1, CK7, and CK19).77–79 Although the surgical outcomes of combined HCC-CC remain indeterminate, intermediate postoperative long-term survival between HCC and ICC has been reported in several studies.80, 81
Pathogenesis and natural history
The pathogenesis of HCC in cirrhosis is a multistep dedifferentiation process that progresses from regenerative nodule to dysplastic borderline nodule to frank HCC. DNs and early HCC are generally asymptomatic and are usually incidental findings on radiographic studies or detected as a result of screening procedures. Early HCC is a usually slow-growing lesion and remains indolent before acquiring oncological features of classical HCC. With stepwise sequence, small HCC acquires progression ability with angiogenesis, increases in tumor size, invades into the vascular structures, and metastasizes within the liver. These microscopic cancer spread process can evolve toward macroscopic formation of vascular tumor thrombi or multiple tumor presentation. HCC is characterized by early development of such intrahepatic metastasis, whereas distant organs are usually involved late in this disease. HCC usually remains asymptomatic until stretching liver capsule, compressing biliary structures, or tumor rupture with increase in size. In some cases, however, hyperbilirubinemia or hypercalcemia is observed without evidence of biliary obstruction or bone metastases as a paraneoplastic syndrome.
Dissemination or extrahepatic metastases occurs in later stages, primarily via the blood stream to the lungs, bones, and brain. The prognosis for patients with extrahepatic disease is dismal with overall survival up to 1 year depending on the extent of tumor involvement and other prognostic factors.
Screening and diagnosis
The primary objective of screening and surveillance for HCC should be to reduce mortality as much as possible in patients who actually develop the cancer and in an acceptably cost-effective manner. Surveillance is not recommended for the general population, given the low incidence of HCC among individuals with no risk factors. Therefore, the first step in HCC screening should be the identification of patients at risk of HCC development. Traditionally, two methodologies have been employed in HCC surveillance for high-risk patients: tumor marker determination, specifically serum alpha-fetoprotein (AFP) concentration, and diagnostic imaging studies.
The American Association for the Study of Liver Disease (AASLD) has established guidelines regarding the use of these screening techniques based on the best existing evidence.82 The AASLD currently recommends serial hepatic ultrasound and serum AFP measurement every 6 to 12 months in at-risk populations (e.g., any patient with cirrhosis). Similarly, the Japanese Society of Hepatology has recommended hepatic ultrasound and serum AFP/plasma des-gamma carboxyprothrombin (DCP) every 3 to 4 months and dynamic CT or MRI every 6–12 months for very high risk population (HBV-related cirrhosis or HCV-related cirrhosis) and hepatic ultrasound and tumor markers every 6 months for high-risk population (HBV-related chronic hepatitis, HCV-related chronic hepatitis, or cirrhosis with other etiologies) with or without dynamic CT or MRI every 6–12 months, as appropriate.83 A randomized-controlled trial from China reported that the use of ultrasound and AFP enable early detection of HCC and lower HCC-related mortality by 37% in hepatitis B patients.84 Various nonrandomized studies also confirmed prognostic improvement through regular surveillance programs among patients at high risk of HCC.85–87 Regarding the interval of screening, recent meta-analysis revealed that every 6 months of surveillance is significantly better than every 12 months of screening in early detection of HCC. Sant et al.88 reported that 70% of newly diagnosed HCC was within Milan criteria (solitary tumor ≤5 cm or ≤3 tumors with each tumor ≤3 cm)89 when screened every 6 months or less, whereas the proportion of HCC within Milan criteria was 57% and the overall survival rate was inferior with surveillance every 6–12 months. When a suspected nodule was detected in the regular screening, a contrast-enhanced dynamic CT or MRI is needed. Because HCC is mainly fed by arterial flow, early enhancement and washout of contrast on the delayed phase of the scan are typical findings suggestive of HCC (Figure 3). These enhancement characteristics increase the specificity of the scan to >95%.90
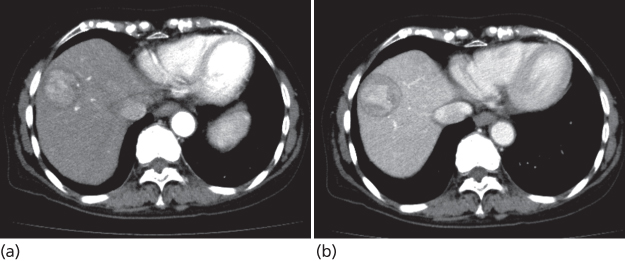
Figure 3 Typical enhancement pattern of hepatocellular carcinoma in dynamic CT scan. Early enhancement in the arterial phase (a) and washout in the late phase (b) are typically observed in moderately differentiated hepatocellular carcinoma.
An update of AASLD recommendations for diagnosis of HCC has been recently published (Figure 4).91 These guidelines suggested that, for patients with cirrhosis, a liver mass detected on a radiographic study does not need biopsy confirmation for the diagnosis of HCC as long as the liver lesion exhibits typical vascular enhancement patterns on dynamic imaging studies with CT scan or MRI for lesions >1 cm in diameter. Lesions <1 cm in diameter should be followed with ultrasound every 3 months. If there is tumor growth or changes in character, further investigation is recommended according to size of tumor.
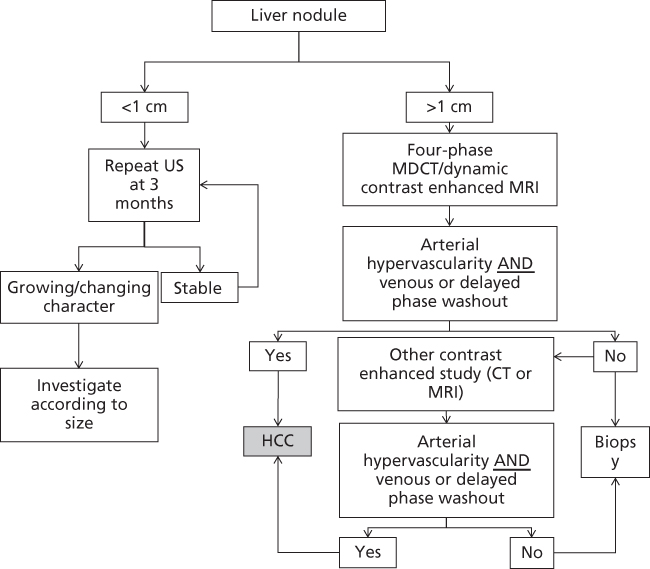
Figure 4 The American Association for the Study of Liver Disease (AASLD) diagnostic algorithm for suspected HCC.
Source: Bruix 2005.82 Reproduced with permission of Wiley.
Staging
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree