PITUITARY ADENOMAS
The role of radiotherapy in the management of pituitary adenomas remains poorly defined because of several factors, including the occasional need for emergent surgical decompression; the availability of competing therapeutic alternatives, such as transsphenoidal adenomectomy and medical treatment with dopamine agonists; the slow decline in hypersecretion after irradiation; the lack of large, well-controlled prospective randomized trials; concerns regarding possible long-term toxic effects of radiotherapy; and the false belief that benign tumors are not effectively treated by radiation therapy.
Available data typically represent experience with a small number of cases accrued over a period of several years or decades and contain the inherent bias of referral patterns at tertiary care centers. Most patients have not been observed for sufficiently long periods to allow adequate interpretation of response and toxicity. Despite these limitations, it has become clear that the role of radiotherapy in the management of pituitary neoplasms falls into two distinct categories: the control of hypersecretion when other modalities have failed or are contraindicated and the control of mass effects. For most pituitary adenomas, external beam irradiation, delivered in conventional fraction sizes of 1.8 to 2 Gy to a total of 45 Gy or more, appears to suffice. Newer techniques—such as radiosurgery, interstitial implantation, and particle beam therapy—are under investigation.
PROLACTINOMAS
Prolactinomas, predominantly macroadenomas, were previously classified with the nonsecreting tumors as chromophobe adenomas; before the prolactin assay era, the major goal of radiotherapy was the control of the mass effect. With the availability of the prolactin assay, it became clear that some macroadenomas are functionally active. Prolactin-secreting microadenomas usually are effectively managed by bromocriptine or, rarely, by transsphenoidal adenomectomy. Long-term control of hyperprolactinemia after surgery alone is rare. Large tumors and tumors that persistently secrete prolactin despite resection can be treated medically with bromocriptine, a dopamine agonist that decreases prolactin levels and causes tumor shrinkage. The continued use of bromocriptine may be complicated by toxic effects, and its discontinuation often results in resumption of tumor growth and of prolactin hypersecretion. Despite prior therapy with bromocriptine, tumor growth may resume in women who become pregnant.
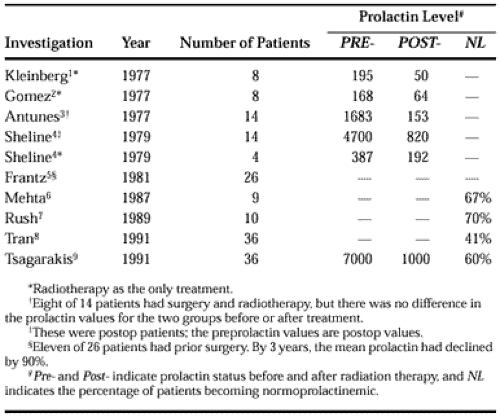
Radiotherapy is indicated if hyperprolactinemia persists despite transsphenoidal tumor resection or the use of bromocriptine. The results of several single-institution studies are summarized in Table 22-1.1,2,3,4,5,6,7,8 and 9 Although irradiation causes a dramatic decrease in prolactin levels in some patients, the response
is not always predictable. Serum assays obtained after radiotherapy have demonstrated a slow and variable decline in prolactin levels. Transient hyperprolactinemia, lasting as long as 2 years, has been described in patients irradiated for other pituitary-hypothalamic conditions.
is not always predictable. Serum assays obtained after radiotherapy have demonstrated a slow and variable decline in prolactin levels. Transient hyperprolactinemia, lasting as long as 2 years, has been described in patients irradiated for other pituitary-hypothalamic conditions.
After irradiation, prolactin levels decrease by an average of 60% by the end of the first year; after 3 or more years, the mean prolactin level decreases to one-tenth of the preirradiation value.5 In another study, 16 (44%) of 36 prolactin-secreting adenomas were controlled after radiotherapy.8 Another 36 female patients (12 with macroprolactinomas and 24 with microprolactinomas) were irradiated to a total dose of 45 Gy (1.8-Gy fractions) with a three-field technique.9 All patients underwent baseline and periodic reassessment of anterior and posterior pituitary function at intervals of 1 year or less while off bromocriptine for at least 2 months; they also had dynamic screening with thyrotropin-releasing hormone and luteinizing hormone–releasing hormone and hypoglycemic stimulation every 2 to 3 years. The preirradiation prolactin levels ranged from 1150 to 34,000 mU/L. With a mean follow-up of 8.5 years (range, 3–14 years), the postirradiation serum prolactin levels fell to normal (i.e., <360 mU/L) in 18 patients (50%). Another 10 patients (28%) had prolactin levels just above the normal range (378–780 mU/L). Only 2 patients (6%) demonstrated an increase in prolactin levels; another patient had a radiographically confirmed recurrence. Neither the pretreatment prolactin level nor the size of the tumor influenced the outcome from radiotherapy.
In a smaller study, long-term control of hyperprolactinemia was achieved in 7 (70%) of 10 patients after a total dose of 45 Gy (1.8-Gy fractions) of radiation.7 These studies elegantly demonstrated that radiotherapy and dopamine agonists are useful for long-term control of subtotally resected macroprolactinomas. Despite the paucity of long-term longitudinal studies of prolactin secretion after radiation therapy, studies indicate that over 2 to 13 years, prolactin levels return to normal or near-normal levels in >75% of irradiated patients.7,9 However, physiologic symptoms, such as amenorrhea from markedly elevated prolactin production, persist even when these levels fall to baseline. For example, in a series of 24 patients with prolactin-secreting macroadenomas treated with transsphenoidal surgery, dopamine-agonist therapy, and 45 Gy radiotherapy, tumor control and prolactin reductions were achieved in all, but amenorrhea persisted in the majority.10
GROWTH HORMONE–SECRETING TUMORS
Initial reports of the effectiveness of radiotherapy for the treatment of growth hormone (GH)–secreting tumors relied on clinical assessments of response, because direct measurements of GH levels were unavailable. Several investigators have reported clinical control rates of 80% to 90% after irradiation to doses of 40 Gy or more using conventional fraction sizes of 1.8 to 2 Gy per day.11,12,13 and 14 Clinical experience also suggests a dose-response relationship; control improves with doses as great as 40 Gy, and toxic effects occur at doses higher than 50 to 54 Gy. In 105 patients with GH-secreting pituitary adenoma treated to a total dose of 42 to 55 Gy, no improvement in local control was found at doses higher than 45 Gy. Moreover, the advent of the serum GH assay revealed that the functional response by these tumors to radiotherapy occurred slowly. The current definition of cure requires a GH level of <5 mU/L (2 ng/mL), with the understanding that long-term survival is dependent on such a reduction. Although radiation-induced GH reduction requires a lag period, as opposed to the immediate decline seen after surgery, the hypothalamic effects of radiation abrogate the endogenous somatostatin tone, thereby abolishing several responses that may enhance GH secretion. In a 20-patient study, arginine increased GH hypersecretion in those with a prior history of acromegaly whose GH levels had normalized after surgery. This phenomenon could not be demonstrated in patients postradiation.15
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree