FDG PET imaging is useful for preoperative diagnosis of pancreatic carcinoma in patients with suspected pancreatic cancer in whom CT fails to identify a discrete tumor mass or in whom FNAs are nondiagnostic. FDG PET imaging is useful for M staging and restaging by detecting CT occult metastatic disease, allowing noncurative resection to be avoided in this group of patients. FDG PET can differentiate post-therapy changes from recurrence and holds promise for monitoring neoadjuvant chemoradiation therapy. The technique is less useful in periampullary carcinoma and marginally helpful in staging except for M staging. As with other malignancies, FDG PET is complementary to morphologic imaging with CT, therefore, integrated PET/CT imaging provides optimal images for interpretation and thus more optimal patient care.
Tumors of the Pancreas
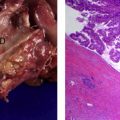
Pancreatic ductal adenocarcinoma is the most common type of pancreatic cancer and arises from the pancreatic ducts. It is the third most common malignant tumor of the gastrointestinal tract and the fifth leading cause of cancer-related mortality accounting for 5% of cancer-related deaths in the United States. Most tumors arise in the head of the pancreas, and patients present with bile duct obstruction, pain, and jaundice. Only 10% to 30% of pancreatic carcinomas are resectable at the time of presentation, the 5-year survival is 18% to 20%, and median survival is 17 to 21 months. Patients with locally advanced nonmetastatic disease have a median survival of 6 to 10 months. Pancreatic carcinoma commonly metastasizes to the liver and patients with metastatic disease have a median survival of 3 to 6 months.
Carcinoma of the ampulla of Vater may be difficult to differentiate from those arising from the head of the pancreas. Ampullary carcinomas have a better prognosis than pancreatic carcinoma because they cause symptomatic biliary obstruction and are diagnosed earlier in the course of the disease.
Acinar cell carcinomas comprise no more than 1% to 2% of all pancreatic cancer, and the prognosis is as poor as for ductal adenocarcinoma.
Cystic neoplasms can arise in the pancreas and differentiation of benign from malignant is critical.
Islet cell tumors and other neuroendocrine (NE) tumors make up a small fraction of all pancreatic neoplasms and are most often located in the body and tail of the pancreas. They are usually slow-growing tumors and are associated with endocrine abnormalities. Endocrine pancreatic tumors include carcinoid tumors, insulinoma that is benign in 90% of patients, gastrinoma, vipoma, and glucagoma that are metastatic at diagnosis in 60% to 80% of patients. The NE tumors will be addressed in another article.
Some of these tumors are associated with elevated serum levels of tumor markers that can be helpful for the diagnosis and surveillance of these patients, such as CA 19-9 for surveillance of patients with pancreatic ductal adenocarcinoma, as well as various peptides for islet cell neoplasms.
The diagnostic issues include early detection, differentiation of malignant from benign tumors (lesion characterization), staging for resection that includes lesion localization, evaluation of proximity to vessels, invasion of adjacent structures, metastasis to regional lymph nodes and distant sites, and assessment of therapeutic response.
Various imaging modalities are available to achieve these goals including ultrasound (US), computed tomography (CT), magnetic resonance imaging (MR imaging), and functional imaging using radiopharmaceuticals (nuclear medicine). Tomographic imaging for functional radioisotopic studies can be performed using single-photon emission tomography technique (SPECT) if the radiopharmaceutical is a single photon emitter, and positron emission tomography technique (PET) if the radiopharmaceutical is a positron emitter.
Anatomical imaging modalities
The suspicion for pancreatic cancer is often raised when either a pancreatic mass or dilatation of the biliary or pancreatic ducts are detected by US or CT.
Transabdominal US is well established as a valuable screening technique that is inexpensive, portable, and sensitive for evaluation of the pancreas, bile duct dilatation, and detection of hepatic lesions as small as 1 cm. It can also provide guidance for biopsy and drainage procedures. Its limitations include poor sensitivity (50%) for detection of small hepatic lesions and regional lymphadenopathy compared with CT and MR imaging.
Endoscopic ultrasound (EUS) is sensitive for the detection choledocolithiasis and pancreatic masses. However, it is highly operator-dependent and requires sedation.
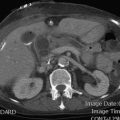
CT is superior to US not only for detection of a pancreatic mass but also for assessment of vascular involvement and invasion of adjacent organs.
For hepatic imaging, CT and MR imaging are based on the dual perfusion of the liver: 80% of the blood flow to normal hepatic parenchyma is derived from the portal vein, whereas nearly all of the blood flow to hepatic neoplasms is derived from the hepatic artery. Therefore some lesions are better seen at different time after intravenous contrast injection. Typically, hypervascular tumors and metastases (hepatocellular carcinoma = HCC, metastases of carcinoid carcinoma, islet cell tumor, malignant pheochromocytoma, renal cell carcinoma, sarcoma, melanoma, and breast carcinoma) may be best seen during the arterial phase of enhancement, or before contrast is administered; whereas hypovascular metastases (colorectal carcinoma, and most metastases of other primaries) are best seen during the portal venous phase of enhancement.
MR imaging is certainly as sensitive as CT for detection of focal hepatic lesions. A multitude of pulse sequences have been developed to characterize lesions. Gadolinium chelate contrast agents are used like the intravenous CT contrast agents, rapidly leaving the vascular space and reaching equilibrium throughout the extracellular fluid compartment after about 3 minutes. Another contrast agent available for MR imaging of the liver is superparamagnetic iron oxide particles (SPIO), a marker of the reticuloendothelial cells (hepatic Kupffer cells). Most malignant tumors do not contain Kupffer cells and have a different T2 signal than normal hepatic parenchyma.
MR cholangiopancreatography (MRCP) permits visualization of the biliary tree noninvasively without the administration of contrast agents. Using a heavily T2-weighted pulse sequence, solid organs and moving fluid have a low signal, whereas relatively stagnant fluid (such as bile) has a high signal intensity, resulting in the biliary tract appearing as a bright well-defined structure. Although MRCP does not provide the resolution of percutaneous transhepatic cholangiography (PTC) or endoscopic retrograde cholangiopancreatography (ERCP), it is able to clearly demonstrate intraluminal filling defects and luminal narrowing. MRCP provides invaluable information in both benign and malignant biliary tract disease.
Cholangiopancreatography via PTC or ERCP is an invasive technique but remains the procedure of choice for high-resolution assessment of the biliary tree anatomy. ERCP is performed by endoscopic cannulation of anatomic tracts and is therefore less invasive than PTC, which requires passage of a needle through the hepatic parenchyma. Contrast material is then injected directly into the biliary tree. Both techniques offer the advantage of allowing interventional procedures such as stent placement in the same setting as the imaging procedure. PTC demonstrates the intrahepatic ducts better than ERCP, which better depicts the extrahepatic ducts.
Anatomical imaging modalities
The suspicion for pancreatic cancer is often raised when either a pancreatic mass or dilatation of the biliary or pancreatic ducts are detected by US or CT.
Transabdominal US is well established as a valuable screening technique that is inexpensive, portable, and sensitive for evaluation of the pancreas, bile duct dilatation, and detection of hepatic lesions as small as 1 cm. It can also provide guidance for biopsy and drainage procedures. Its limitations include poor sensitivity (50%) for detection of small hepatic lesions and regional lymphadenopathy compared with CT and MR imaging.
Endoscopic ultrasound (EUS) is sensitive for the detection choledocolithiasis and pancreatic masses. However, it is highly operator-dependent and requires sedation.
CT is superior to US not only for detection of a pancreatic mass but also for assessment of vascular involvement and invasion of adjacent organs.
For hepatic imaging, CT and MR imaging are based on the dual perfusion of the liver: 80% of the blood flow to normal hepatic parenchyma is derived from the portal vein, whereas nearly all of the blood flow to hepatic neoplasms is derived from the hepatic artery. Therefore some lesions are better seen at different time after intravenous contrast injection. Typically, hypervascular tumors and metastases (hepatocellular carcinoma = HCC, metastases of carcinoid carcinoma, islet cell tumor, malignant pheochromocytoma, renal cell carcinoma, sarcoma, melanoma, and breast carcinoma) may be best seen during the arterial phase of enhancement, or before contrast is administered; whereas hypovascular metastases (colorectal carcinoma, and most metastases of other primaries) are best seen during the portal venous phase of enhancement.
MR imaging is certainly as sensitive as CT for detection of focal hepatic lesions. A multitude of pulse sequences have been developed to characterize lesions. Gadolinium chelate contrast agents are used like the intravenous CT contrast agents, rapidly leaving the vascular space and reaching equilibrium throughout the extracellular fluid compartment after about 3 minutes. Another contrast agent available for MR imaging of the liver is superparamagnetic iron oxide particles (SPIO), a marker of the reticuloendothelial cells (hepatic Kupffer cells). Most malignant tumors do not contain Kupffer cells and have a different T2 signal than normal hepatic parenchyma.
MR cholangiopancreatography (MRCP) permits visualization of the biliary tree noninvasively without the administration of contrast agents. Using a heavily T2-weighted pulse sequence, solid organs and moving fluid have a low signal, whereas relatively stagnant fluid (such as bile) has a high signal intensity, resulting in the biliary tract appearing as a bright well-defined structure. Although MRCP does not provide the resolution of percutaneous transhepatic cholangiography (PTC) or endoscopic retrograde cholangiopancreatography (ERCP), it is able to clearly demonstrate intraluminal filling defects and luminal narrowing. MRCP provides invaluable information in both benign and malignant biliary tract disease.
Cholangiopancreatography via PTC or ERCP is an invasive technique but remains the procedure of choice for high-resolution assessment of the biliary tree anatomy. ERCP is performed by endoscopic cannulation of anatomic tracts and is therefore less invasive than PTC, which requires passage of a needle through the hepatic parenchyma. Contrast material is then injected directly into the biliary tree. Both techniques offer the advantage of allowing interventional procedures such as stent placement in the same setting as the imaging procedure. PTC demonstrates the intrahepatic ducts better than ERCP, which better depicts the extrahepatic ducts.
PET
18 F-Fluorodeoxyglucose (FDG)
Although variations in uptake are known to exist among tumor types, elevated uptake of 18 F-fluorodeoxyglucose (FDG) has been demonstrated in most primary malignant tumors. This is because of the expression of increased numbers of glucose transporter proteins and increased intracellular enzyme levels of hexokinase and phosphofructokinase, among others, which promote glycolysis. Pancreatic carcinoma does overexpress Glut-1. Therefore, FDG PET imaging can be used to exploit the metabolic differences between benign and malignant cells for imaging purposes.
Improvements in the distribution of FDG by commercial companies and the widespread oncologic applications including differentiation of benign from malignant lesions, staging malignant lesions, detection of malignant recurrence, and monitoring therapy have contributed to the establishment of the PET technology in many medical centers in the United States and Europe and progressively throughout the world. Because of the limitations of FDG related to variations of physiologic uptake and overlap of uptake between inflammatory and malignant lesions, other PET radiopharmaceuticals have been investigated for clinical use.
Instrumentation for Molecular Imaging with PET
The clinical utility of FDG imaging was first established using dedicated PET tomographs equipped with multiple rings of bismuth germanate oxide (BGO) detectors, but a spectrum of equipment is now available for positron imaging including gamma camera-based PET at the low end of the spectrum and dedicated PET tomographs equipped with newer detector materials. The advantages and limitations of each of these systems is beyond the scope of this review.
Although numerous studies have shown that the sensitivity and specificity of FDG imaging is superior to that of CT in many clinical settings, the inability of FDG imaging to provide accurate anatomic localization remains a significant impairment in maximizing its clinical utility. Because FDG is a tracer of glucose metabolism, its distribution is not limited to malignant tissue. To avoid misinterpretations, the interpreter must be familiar with the normal pattern and physiologic variations of FDG distribution and with clinical data relevant to the patient. It is also important to standardize the environment of the patient during the uptake period so as to limit physiologic variations of FDG uptake.
The limitations of anatomic imaging with CT and MR imaging are related to size criteria for differentiation of benign from malignant lymph nodes, difficulty differentiating post-therapy changes from tumor recurrence, and difficulty differentiating non-opacified loops of bowel from metastases in the abdomen and pelvis.
Close correlation of FDG studies with conventional CT scans helps to minimize these difficulties. Interpretation has been traditionally accomplished by visually correlating FDG and CT images. In 2000, integrated PET/CT imaging systems became available commercially allowing optimal coregistration of images and became rapidly the standard of care. Integrated SPECT/CT systems are also available and are becoming the standard of care when anatomic localization of SPECT anomalies is critical.
The CT portion of the study is most commonly acquired with low-mAs to reduce the radiation dose to the patient, although it can be acquired with diagnostic CT protocols. The reduced-dose CT is used for attenuation correction and anatomic localization with the help of the fusion of anatomic and molecular images. The incremental value of integrated PET/CT images compared with PET alone, or PET correlated with a CT obtained at a different time conclude the following: (1) improvement of lesion detection on both CT and FDG PET images, (2) improvement of the localization of foci of FDG uptake resulting in better differentiation of physiologic from pathologic uptake, and (3) precise localization of the malignant foci, for example in the skeleton versus soft tissue, or liver versus adjacent bowel or node. PET/CT fusion images affect the clinical management by guiding further procedures, excluding the need of further procedures, and changing both inter- and intramodality therapy. For example, precise localization of metastatic lymph nodes could result in a less invasive and more efficient surgical procedure. PET/CT fusion images have the potential to provide important information to guide the biopsy of a mass to more metabolically active regions of the tumor and to provide better maps than CT alone to modulate field and dose of radiation therapy.
Integrated PET/CT and SPECT/CT imaging with integrated systems may be especially important in the abdomen and pelvis owing to the paucity of anatomic landmarks on functional imaging studies. For example, FDG PET images alone may be difficult to interpret owing to the absence of anatomic landmarks (other than the liver, kidneys, and bladder); the presence of nonspecific uptake in the stomach, small bowel, and colon; and ureteral activity of FDG. Images of the abdomen and pelvis should be obtained with the arms elevated, whenever possible, to avoid artifacts due to motion and to beam hardening. A review of PET/CT for gastrointestinal tumors has been published.
PET and PET/CT for the evaluation of pancreatic carcinoma
The difficulty in correctly determining a preoperative diagnosis of pancreatic carcinoma is associated with two types of adverse outcomes. First, less aggressive surgeons may abort attempted resection because of a lack of tissue diagnosis. This is borne out by the significant rate of “reoperative” pancreaticoduodenectomy performed at major referral centers. In a review of the M.D. Anderson Cancer Center involving 29 patients undergoing successful pancreaticoduodenectomy after failure to resect at the time of initial laparotomy, 31% did not undergo resection at the time of the initial procedure because of the lack of tissue confirmation of malignancy. A second type of adverse outcome generated by failure to obtain a preoperative diagnosis occurs when more aggressive surgeons inadvertently resect benign disease. This is particularly notable in those patients who present with suspected malignancy without an associated mass on CT scan, occurring in up to 55% of patients.
Preoperative Diagnosis of Pancreatic Carcinoma
Anatomical imaging modalities for diagnosis of pancreatic carcinoma
The reported diagnostic accuracy of CT for detection of pancreatic cancer is in the 85% to 95% range. The sensitivity and positive predictive value of dual-phase CT protocols for detection of pancreatic tumors are 97% and 92% respectively. Interpretation of the CT scan is sometimes difficult in the setting of mass-forming pancreatitis or other questionable findings, such as enlargement of the pancreatic head without definite signs of malignancy. The diagnostic performance of MR imaging remains similar to that of CT. Even with the latest technology improvements, MR imaging demonstrates sensitivity of 86% and specificity of 89% for detection of pancreatic tumors.
In a study of 80 patients with cancer, the sensitivity of EUS (98%) for detecting a pancreatic mass was greater than that of CT (86%). In addition, EUS offers the possibility of tissue diagnosis with ultrasound-guided fine needle biopsy (FNA) with reported diagnostic yield of 68% and diagnostic accuracy of 74%. The reported overall diagnostic rate, sensitivity, and negative predictive value of FNA biopsies guided using CT (98%, 95%, and 60%, respectively) are not significantly different from those guided using EUS (88.9%, 85%, and 57.%, respectively).
The accuracy of ERCP is 80% to 90% for differentiation of benign from malignant pancreatic processes, including differentiation of tumor from chronic pancreatitis, because of the high degree of resolution of ductal structures that ERCP provides. The limitations of ERCP include false negatives when the tumor does not originate from the main duct, a 10% technical failure rate, and up to 8% morbidity (primarily iatrogenic pancreatitis). Principal advantages of ERCP include the ability to perform FNA biopsy and other interventional procedures (eg, sphincterotomy or stent placement). Although FNA biopsy may provide a tissue diagnosis, this technique suffers from significant sampling error.
18 F-FDG PET/CT for the preoperative diagnosis of pancreatic carcinoma
PET has a role in establishing the diagnosis of pancreatic carcinoma when the CT is nondiagnostic, when the biopsy is equivocal or nondiagnostic, when there is concurrent chronic pancreatitis, and for cystic lesions of the pancreas, even with the limitations of FDG PET imaging discussed in the section on limitations.
The summary of the literature published in 2001 reported average sensitivity and specificity of 94% and 90%, respectively. All studies included have reported relatively high rates of sensitivity (85% to 100%), specificity (67% to 99%), and accuracy (85% to 93%) for 18 F-FDG PET imaging in the differentiation of benign from malignant pancreatic masses, and most suggest improved accuracy compared with CT. These results are similar to the findings in the series of Rose and colleagues with a sensitivity of 92% and specificity of 85% for FDG PET compared with 65% and 62% respectively for CT imaging. FDG PET was particularly helpful in patients without a definite mass on CT and with nondiagnostic FNA. A recent review by Pakzad and colleagues suggested that the overall sensitivity of FDG PET for detection of pancreatic carcinoma varies between 90% and 95% and specificity from 82% to 100%.
The performance of FDG PET was compared with CT and EUS for the diagnosis and staging of pancreatic cancer in 35 patients. For the diagnosis, the sensitivity of EUS was 93%, compared with 87% for FDG PET and 53% for CT. EUS-guided FNA allowed tissue diagnosis in 67% of the patients.
FDG PET is more accurate than conventional imaging techniques (CT and MR) for differentiating benign from malignant cystic lesions of the pancreas and intraductal papillary mucinous tumors (IPMT). In a prospective study of 50 patients with suspected cystic pancreatic tumors, the sensitivity, specificity, positive and negative predictive value, and accuracy of FDG PET for detection of malignant tumors were 94%, 94%, 89%, 97%, and 94% compared with 65%, 88%, 73%, 83%, and 80% for CT. In a series of 64 patients with suspected IPMT, the sensitivity of FDG PET was 80% (4/5) for carcinoma in situ and 95% (20/21) for invasive carcinoma, both superior to CT or MRCP, which were strongly suggestive of invasive carcinoma in only 62% of patients who had invasive carcinoma. FDG uptake was absent in all adenomas (n = 13) and 87% (7/8) of borderline IPMNs. A positive FDG PET influenced the management of 10 patients with malignant IPMNs.
As for other malignancies, studies on a small number of patients suggest that the degree of FDG uptake in pancreatic malignancies correlates with the prognosis. Nakata and colleagues noted an inverse correlation between standard uptake value (SUV) and survival in 14 patients with pancreatic adenocarcinoma. Patients with an SUV greater than 3.0 had a mean survival of 5 months compared with 14 months in those with an SUV less than 3.0. Zimny and colleagues performed a multivariate analysis on 52 patients, including SUV and accepted prognostic factors, to determine the prognostic value of FDG PET. The median survival of 26 patients with SUV greater than 6.1 was 5 months compared with 9 months for 26 patients with SUV less than 6.1. The multivariate analysis revealed that SUV and Ca 19-9 were independent factors for prognosis. Another study of 118 patients demonstrated that survival was significantly influenced by tumor stage, tumor grade, and SUV.
Together, these series support the conclusion that FDG PET imaging may represent a useful adjunctive study in the evaluation of patients with suspected pancreatic cancer, especially when CT imaging results are inconclusive and/or FNA is nondiagnostic or there are cystic components.
Other PET tracers for diagnosis of pancreatic carcinoma
A pilot study of five patients compared 18 F-fluoro-L-thymidine (FLT) and FDG for detection of primary pancreatic adenocarcinoma, 18 F-FLT PET/CT scanning showed poor lesion detectability and relatively low levels of radiotracer uptake in the primary tumor compared with FDG.
11 C-acetate does accumulate physiologically in the pancreas, allows rapid metabolic imaging using PET, and may be a useful metabolic probe for the study of pancreatic physiology and disease. However, adenocarcinoma of the pancreas demonstrated no significant uptake of 11 C-acetate.
Staging of Pancreatic Carcinoma
In the TNM staging system for pancreatic cancer, Stage I disease is confined to the pancreas. Stage II disease is characterized by extrapancreatic extension (T stage), Stage III by lymph node involvement (N stage), and Stage IV by distant metastases (M stage) ( Tables 1 and 2 ).
| T (Tumor) | |
| TX | Primary tumor cannot be assessed. |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor is ≤2 cm in greatest dimension and confined to pancreas. |
| T2 | Tumor is >2 cm and confined to pancreas. |
| T3 | Tumor extends beyond pancreas but does not involve celiac axis or superior mesenteric artery. |
| T4 | Primary tumor involves either celiac axis or superior mesenteric artery. |
| N (Nodal involvement) | |
| NX | Regional lymph nodes cannot be assessed. |
| N0 | No regional lymph node metastasis. |
| N1 | Regional lymph node metastasis. |
| M (Metastases) | |
| MX | Distant metastasis cannot be assessed. |
| M0 | No distant metastases. |
| M1 | Distant metastasis. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree