The rapid growth of minimally invasive technology and experience in recent decades has revolutionized many aspects of oncologic surgery. Adoption of laparoscopic pancreatectomy has been slow due to the inherent anatomic complexity of pancreatic surgery, as well as concerns of perioperative complications and compromised oncologic results. With increasing surgeon experience and growing data, laparoscopic pancreatic resection is generating considerable attention and enthusiasm. This article provides an overview of laparoscopic pancreatic tumor surgery with respect to tumor biology and technical approaches. Current applications of laparoscopic approaches to left pancreatectomy, tumor enucleation, central pancreatectomy, and pancreaticoduodenectomy for treatment of pancreatic tumors are considered in light of available evidence demonstrating feasibility, safety, and oncologic efficacy. Future directions in minimally invasive pancreatic surgery are explored.
Over the last 3 decades, the exponential growth of minimally invasive technology has facilitated the development of laparoscopic techniques and thereby revolutionized many aspects of abdominal surgery. Laparoscopic solid organ resection is now widely accepted in endocrine, urologic, and colorectal surgery. Until recently, however, other organs such as the esophagus, stomach, liver, biliary tract, and pancreas have received little attention as targets of laparoscopic resection. Reasons for the lag of laparoscopic resection for these sites are multifactorial; beyond operative complexity, the majority of surgeons trained to operate on these organs traditionally had little experience with advanced laparoscopic techniques.
Progress with laparoscopic pancreatic resections was particularly slow, owing to the organ’s retroperitoneal location, proximity to major vasculature structures, and propensity for fistula formation. The first reports of laparoscopic intervention for pancreatic surgery came from Germany and the United Kingdom in the 1970s, mainly for the diagnosis and staging of pancreatic cancers. Gagner and colleagues published the first series of laparoscopic resection for distal pancreatic tumors in 1996, though persistent concerns for postoperative morbidity, adequacy of resection, and port-site recurrence delayed adoption of laparoscopic pancreatectomy.
Since then, improvements in technology, increase in expertise, and validation of improved perioperative outcomes (decreased postoperative length of stay, narcotic requirement, and cost) for other major abdominal operations have stimulated increased interest in laparoscopic pancreatic surgery. This article describes the various applications of laparoscopy for oncologic pancreatic surgery with a review of recent evidence regarding outcome. In addition, consideration is given to pancreatic tumor biology and associated implications for laparoscopic management. Finally, future directions in minimally invasive pancreatic surgery are discussed.
Laparoscopic applications for management of pancreatic tumors
Left (Distal) Pancreatectomy
Technical considerations
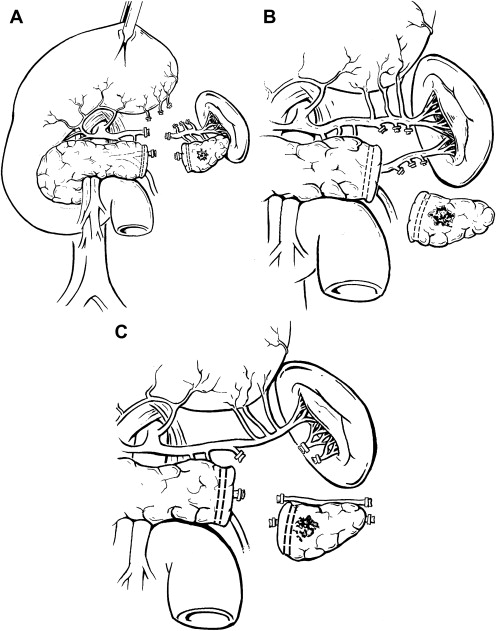
At present, the most common type of pancreatic resection performed laparoscopically is the left (distal) pancreatectomy ( Fig. 1 A–C). Although in general Whipple resections are more frequently performed, left pancreatectomy is inherently more suited to minimally invasive techniques as no reconstruction is typically required. The authors prefer the term “left” pancreatectomy, as the volume of pancreatic parenchyma resected to the left of the portal-mesenteric venous axis is variable depending on tumor size and location within the gland.
The steps of laparoscopic left pancreatectomy are described in detail elsewhere, though technical variability is common based on surgeon preference and patient factors ( Fig. 2 A–D). Some surgeons prefer the right lateral decubitus patient position, as it affords excellent visualization of the lesser sac with natural gravity retraction of the spleen, stomach, and colon. Others favor the supine position and typically place an additional port for gastric retraction. A periumbilical camera port is usually placed with additional trocars in the left abdomen. In cases of malignancy, initial laparoscopic surveillance for metastatic disease is performed. Once the lesser sac is entered by division of the greater omentum below the gastroepiploic vessels, the stomach falls into the right upper quadrant and the ventral pancreatic surface is exposed.
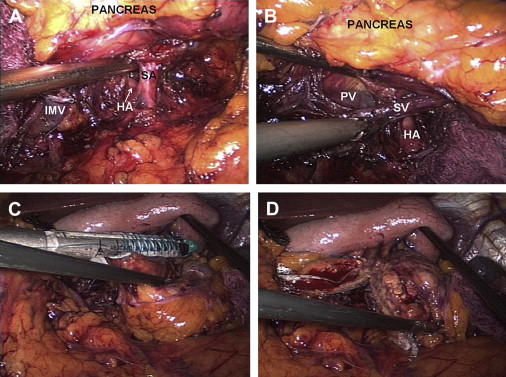
Dissection is typically performed using bipolar electrocautery or ultrasonic coagulating shears. The short gastric vessels are left intact if splenic preservation is planned, especially if the Warshaw technique of splenic vessel ligation is performed ( Fig. 1 C). Depending in part on tumor location, dissection is performed in medial-to-lateral or lateral-to-medial fashion along the inferior border of the pancreas to delineate the splenic vein with division of its pancreatic branches. The authors prefer to divide the inferior mesenteric vein early, to avoid inadvertent injury to this structure with resultant hemorrhage. The splenic artery is then identified superior to or posterior to the pancreas. The superior approach is relatively straightforward and safe, as the vessel courses along the superior aspect of the gland. While the posterior approach ( Fig. 2 A, B) can provide excellent access to the origin of the splenic artery, care must be taken to avoid transecting the main trunk of the celiac axis or common hepatic artery. When splenic vessels are sacrificed, division can be performed using endoscopic staplers as well as clip and suture ligation.
Some surgeons favor the use of hand-access ports whereas others prefer the straight laparoscopic technique. The authors reserve use of the hand-access technique for more complex cases or those with larger tumors. The use of hand-assist ports provides numerous advantages for the surgeon in palpation of tumor, surrounding structures, as well as arterial pulsations to guide dissection and transection. Manual retraction and digital pressure to arrest hemorrhage are also facilitated. Placement of hand ports have been described in the supraumbilical and infraumbilical midline as well as the right lower quadrant. Decision to use hand access is based on a combination of specimen size, tumor location, patient habitus, and surgeon preference. However, it is unknown whether the theoretical advantages of hand-assist resections translate into improved patient outcomes, as direct comparisons between complete-laparoscopic and hand-assisted techniques are lacking. A single-institution analysis of 17 patients who underwent laparoscopic pancreatectomy with hand assistance revealed acceptable morbidity (25%) and favorable length of hospital stay (mean 5.5 days) in comparison with published historical data from open pancreatectomy.
Pancreatic fistula and stump management
Pancreatic fistula is a well-recognized complication of pancreatectomy. Clinical manifestations of postoperative pancreatic fistula range from asymptomatic amylase-rich fluid in surgical drains to life-threatening sepsis requiring reoperation. The International Study Group on Pancreatic Fistula Definition (ISGPF) provided a useful classification improving uniformity in recent literature. In open left pancreatectomy, the overall rate of fistula formation generally ranges between 5% and 18%. Management of the pancreatic stump, the presumed source of leak after left pancreatectomy, has therefore received much attention, with varied proponents of main duct suture ligation, oversewing of the pancreatic stump, linear stapled closure with or without staple-line reinforcement, electrocautery, ultrasound coagulation, omental patch, fibrin glue application, enteric anastomosis, octreotide administration, or various combinations of the listed techniques. In-line radiofrequency ablation is another effective, yet expensive option for managing the pancreatic stump.
Comparison studies of these techniques provide mixed results, with some analyses identifying reduced fistula rates after stapling, some reporting more favorable results using hand-suturing, and still others showing no difference between the 2 methods. The multi-institutional DISPACT trial, an ongoing randomized controlled study, aims to compare hand-sewn and stapled techniques in left pancreatectomy. Other investigators have evaluated the utility of staple-line reinforcement. A recent series of patients who had transection with staple-line reinforcement (n = 29) compared with ones that did not (n = 23) suggested a 84% reduction in pancreatic fistula when reinforcement was used ( P = .04). However, another retrospective institutional assessment found no difference in rate of fistula formation after stapled transection with (n = 45) or without (n = 41) staple-line reinforcement (33% vs 24%, P >.05). In turn, Bilimoria and colleagues suggested that perhaps more important than the method of transection is the identification and selective suture ligation of the main pancreatic duct; in their analysis of 126 patients over a span of 9 years, fistula rates were 9.6% in the 74 cases of duct ligation and 34% in the 53 cases without duct ligation ( P <.001). Absence of selective ligation was the only clinicopathologic and operative factor that maintained association with increased risk of pancreatic fistula development (odds ratio 5.0, 95% confidence interval 2.0–10.0). Some have suggested that selective ligation may be more difficult to achieve during laparoscopic resection, and may therefore contribute to increased fistula rates. Most of the mentioned studies evaluate stump management in the setting of open resection, leaving extrapolation of conclusions to the laparoscopic approach. Thus, prospective investigations in laparoscopic left pancreatectomy are necessary.
As fistula formation after left pancreatectomy is relatively common, concerns of inadequate laparoscopic management of the pancreatic stump contributed to the slow adaptation of minimally invasive pancreatic resection. Nevertheless, comparison of the open and laparoscopic approaches has not shown increased rates of fistula formation after laparoscopic resection. In the largest published study of laparoscopic and open left pancreatectomy, 142 laparoscopic and 200 case-matched open cases were compared, demonstrating similar overall fistula rates (26% laparoscopic vs 32% open, P = .28). When only clinically significant fistulas were considered (ISGPF grades B/C), there was a nonsignificant trend toward increased fistula formation in open pancreatectomy (11% laparoscopic vs 18% open, P = .10). In a recent single-institution Korean study evaluating 93 laparoscopic and 35 open cases, fistula formation rates were similar at 8.6% and 5%, respectively ( P = .32).
Splenic preservation
When anatomically and biologically possible, splenic preservation during left pancreatectomy has been shown to be associated with reduced postoperative overall and infectious complications. Proportion of cases with splenic preservation is greater for those patients having laparoscopic left pancreatectomy than those having open resections in the 2 largest comparative series to date (40.8% vs 5.7% and 30% vs 12%). Proponents of the laparoscopic approach support improved visualization of the lesser sac and retropancreatic space as provided by angled cameras, and some have attributed the increased rates of splenic preservation to this and the inherent magnification associated with laparoscopy.
Spleen-preserving left pancreatectomy is usually a tedious procedure whereby division of the multiple small arterial and venous connections between the pancreas and the splenic vessels is performed ( Fig. 1 B). In this manner, the main splenic blood supply is preserved as is splenic function. In 1988, Warshaw described a more expedient technique of splenic preservation whereby the splenic vessels are ligated and divided at both the pancreatic transection line and at the splenic hilum (see Fig. 1 C). In this approach, splenic viability is maintained via the short gastric vessels. Splenic infarction and gastric variceal hemorrhage are concerns of using this approach. Furthermore, preservation of splenic function is not certain when the organ is supported solely by the short gastric vessels. Long-term studies evaluating splenic function and value of splenic preservation are lacking.
Comparison with open left pancreatectomy
Numerous reports of institutional experiences with laparoscopic left pancreatectomy have been published in the last decade. Table 1 summarizes series incorporating at least 25 patients. The majority of these series demonstrate feasibility of laparoscopic left pancreatectomy with acceptable morbidity and minimal mortality. To date, few studies have systematically compared laparoscopic and open approaches with regard to postoperative outcomes.
| Study | Cases | Multi-Institutional | Mean Operative Time (Minutes) | Mean Blood Loss (mL) | Mean Length of Stay (Days) | Conversion Rate (%) | Splenic Preservation (%) | Overall Morbidity (%) | Pancreatic Fistula Rate (%) | Mortality (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Park and Heniford, 2002 | 25 | Yes | 222 | 274 | 4 | 8 | 48 | 16 | 4 | 0 |
| Mabrut et al, 2005 | 96 | Yes | 200 a,c 195 b,c | NR | 7 | 10 | 71 | 53 | 16 | 0 |
| Melotti et al, 2007 | 58 | Yes | 165 c | NR | 9 c | 0 | 55 | 53 | 27.5 | 0 |
| Eom et al, 2008 | 31 | No | 218 | NR | 11.5 | 0 | 42 | 36 | 10 | 0 |
| Fernandez-Cruz et al, 2007 | 82 | No | NR | NR | 7 c | 7 | 64 | 20 | 9 | 0 |
| Taylor et al, 2008 | 46 | Yes | 157 c | 200 c | 7 c | 26 | 48 | 39 | 15 | 0 |
| Laxa et al, 2008 | 32 | Yes | 238 | 221 | 5 | 6 | 19 | 34 | 19 | 0 |
| Sa Cunha et al, 2008 | 31 | No | 200 a,c 246 b,c | 100 c,d | 12.7 d | 19 | 80 | 31 d | 20 a 45 b | 0 |
| Kooby et al, 2008 | 167 | Yes | 230 | 357 | 5.9 | 13 | 31 | 40 | 11 | 0 |
c Median reported rather than mean.
d Reported for all laparoscopic pancreatic resections in series, not individually reported for laparoscopic left pancreatectomy.
Comparative analyses between laparoscopic and open left pancreatectomy are summarized in Table 2 . Two types of outcomes deserve attention: perioperative and long-term. The primary perioperative outcomes can be further divided into patient-related, such as pain assessments, blood loss, operative time, complication profiles, and hospital length of stay; or disease-related, such as tumor margins and node assessments in cases of malignancy. Long-term outcomes primarily relate to malignant disease, and these include tumor recurrence and patient survival. Given the relative infrequency of left pancreatic resection for cancer, meaningful long-term cancer outcomes are difficult to capture; therefore, most existing data to date focus on perioperative results.
| Study | Cases | Mean Operative Time (Minutes) | Mean Blood Loss (mL) | Splenic Preservation (%) | Mean Length of Stay (Days) | Overall Morbidity (%) | Fistula Rate (%) | Mortality (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LLP | OLP | LLP | OLP | LLP | OLP | LLP | OLP | LLP | OLP | LLP | OLP | LLP | OLP | LLP | OLP | |
| Velanovich, 2006 a , | 15 | 15 | NR | NR | NR | NR | 0 | 0 | 5.0 b | 8.0 b | 20 | 27 | 13 | 13 | 0 | 0 |
| Misawa et al, 2007 | 8 | 9 | 255 b | 205 b | 14 b | 307 b | 12.5 | 0 | 10.0 b | 16.0 b | NR | NR | 0 | 22 | 0 | 0 |
| Teh et al, 2007 | 12 | 16 | 278 | 212 | 193 | 609 | 62 | 17 | 6.2 | 10.6 | 17 | 56 | 8 | 6 | 0 | 0 |
| Eom et al, 2008 a , | 31 | 62 | 218 | 195 | NR | NR | 42 | NR | 11.5 | 13.5 | 36 | 24 | 9.7 | 6.5 | 0 | 0 |
| Kim et al, 2008 | 93 | 35 | 195 b | 190 b | 110 | 110 | 40.8 | 5.7 | 10 b | 16 b | 25 | 29 | 8.6 | 14.3 | 0 | 0 |
| Matsumoto et al, 2008 | 14 | 19 | 291 | 213 | 247 | 400 | 7 | NR | 12.9 | 23.8 | NR | NR | 0 | 10.5 | 0 | 0 |
| Kooby et al, 2008 a , | 142 | 200 | 230 | 216 | 357 | 588 | 30 | 12 | 5.9 | 9.0 | 40 | 57 | 11 | 18 | 0 | 1 |
| Nakamura et al, 2009 | 21 | 16 | 308 | 282 | 249 | 714 | 35 | 31 | 10.0 | 25.8 | 0 | 19 | 0 | 12.5 | 0 | 0 |
Review of trends from comparison studies between laparoscopic and open left pancreatectomy reveals that in general, despite equivalent or somewhat increased operative time, the laparoscopic technique is associated with a reduction in intraoperative blood loss and postoperative length of stay. Overall perioperative morbidity and mortality seem similar with both approaches (see Table 2 ). Studies have also identified reduced time to return of bowel function, oral intake, and ambulation as well as decreased narcotic requirements as advantages of minimally invasive left pancreatectomy. Although these observations parallel cited benefits of the laparoscopic technique in other abdominal operations, selection bias still remains a limitation, which can only be addressed through randomized controlled studies. These existing data suggest that when compared with the open approach, laparoscopic left pancreatectomy is feasible and safe, and may be associated with reduced blood loss, shorter hospital length of stay, reduced postoperative pain, and greater likelihood of splenic preservation.
Pancreatic Enucleation
Technical considerations
Some benign lesions and low-grade malignancies of the pancreas may be suitable for enucleation. In this approach, the lesion is shelled out and the surrounding pancreatic parenchyma is preserved. Key elements of consideration are tumor size, biology, and location with respect to the main pancreatic duct. Smaller, encapsulated tumors with indolent behavior (such as insulinoma), located away from the main duct, are ideal for this approach.
The first reports of laparoscopic pancreatic enucleation were published by Amikura and colleagues in 1995 for treatment of an adrenocorticotropic hormone-producing pancreatic tumor and Gagner and colleagues in 1996 for insulinoma. Since then, several small series have been published, mostly reporting on solitary insulinomas, the most commonly occurring functional pancreatic neuroendocrine tumor ( Table 3 ). Enucleation can be appropriate for cystic lesions with low malignant potential as well. The technique is typically applied to lesions in the body and tail, though superficial tumors of the anterior head and neck have also been successfully enucleated.
| Study | Cases | Pathology | Conversion Rate (%) | Mean Tumor Size (cm) | Tumor Location | Use of Intraoperative Ultrasound (%) | Mean Operative Time (Minutes) | Mean Blood Loss (mL) | Mean Length of Stay (Days) | Overall Morbidity (%) | Pancreatic Fistula Rate (%) | Mortality (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patterson et al, 2001 | 4 | Islet cell tumors | NR | NR | Body and tail | 100 | NR | NR | NR | NR | 25 | 0 |
| Ayav et al, 2005 | 19 | Insulinomas | 10.5 | 1.5 | 4 head; 1 isthmus; 9 body; 3 tail | NR | 115 | NR | NR | NR | 42 | 0 |
| Mabrut et al, 2005 | 22 | NR | 4.5 | NR | NR | NR | 120 a | NR | NR | NR | 29 | 0 |
| Toniato et al, 2006 | 4 | Insulinomas | NR | NR | NR | 100 | NR | NR | NR | NR | NR | 0 |
| Liu et al, 2007 | 9 | Insulinomas | 33.3 | 1.5 b | 4 body; 2 tail | NR | 159 b | 77 b | 11.8 b | 28 b | 17 | 0 |
| Schraibman et al, 2007 | 5 | Insulinomas | 0 | 1.3–2.0 | Body and tail | NR | 130 | “Minimal” | 3 | NR | 0 | 0 |
| Sweet et al, 2007 | 9 | Insulinomas | 22.2 | NR | NR | 66.7 | NR | NR | 2.3 | NR | 77.8 | 0 |
| Fernandez-Cruz et al, 2008 | 21 | 15 insulinoma; 6 nonfunctioning PNET | 4.8 | 3.0 | 4 head; 1 neck; 12 body and tail | NR | 120 | <220 | 5.5 | 42.8 | 38 | 0 |
| Roland et al, 2008 | 10 | Insulinomas | 20 | NR | NR | NR | NR | NR | NR | NR | 12.5 | 0 |
| Luo et al, 2009 | 18 | Insulinomas | 11.1 | NR | 4 head; 1 uncinate; 2 neck; 9 body | NR | 85 a | 255 a | NR | NR | 25 | 0 |
| Isla et al, 2009 | 14 | Insulinomas | 7.1 | NR | 4 head; 6 body; 3 tail | NR | NR | NR | NR | NR | 7.7 | 0 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree