Key points
- •
Percutaneous musculoskeletal biopsy planning should involve multidisciplinary collaboration
- •
Recognize common “pseudotumors” and “don’t-touch lesions”
- •
A solitary bone lesion of unknown etiology is a primary tumor until proven otherwise
- •
Perform the biopsy with imaging modality that best demonstrates the lesion
- •
The biopsy needle path should:
- •
Be aligned with the proposed surgical incision
- •
Be confined to the anatomic compartment involved
- •
Not cross joint spaces, tendons, ligaments, or neurovascular structures
- •
Background
Percutaneous, image-guided musculoskeletal biopsy (PMB) is a well-established, safe, and accurate procedure. It has significantly reduced the need for open surgical biopsy—a more invasive and less cost-effective procedure that is associated with a higher complication rate, longer recovery time, and longer time to initiation of therapy. Historically, clinicians have been reluctant to refer their patients for image-guided percutaneous biopsy following reports of diagnostic errors, complications, and unnecessary amputations, particularly if biopsies were performed at centers not specialized in orthopedic oncology. It is now widely accepted that the assessment and biopsy of musculoskeletal lesions should be performed at the institution where the lesion can be definitively managed. Unlike other percutaneous biopsies performed by radiologists, the approach used for biopsy of musculoskeletal lesions, particularly those in the extremities, needs to be closely aligned with the planned surgical approach.
An additional challenge relates to the ever-increasing demand for high-quality tissue samples. Targeted drug therapy for each unique genetic profile is one of the primary goals of a personalized model of patient care. The histologic and biologic heterogeneity of tumors has significant implications for the molecular profiling of cancers, including the ability to identify specific tumor markers and predict the response to targeted therapies. Changes in tumor biology during therapy need to be characterized in patients with disease progression prior to commencing second- and third-line agents. In those with metastatic disease, treatment should be based on the “metastatic” tumor biology, rather than that of the initial diagnostic specimen from the primary lesion. Communication between the interventional radiologist and the orthopedic pathologist is essential to ensure that the appropriate types, quantity, and quality of samples are acquired.
This chapter reviews the approach to percutaneous image-guided biopsy of musculoskeletal lesions. The various techniques and devices are outlined, and the merits, limitations, and complications of each are discussed.
Indications and contraindications
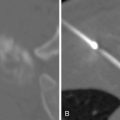
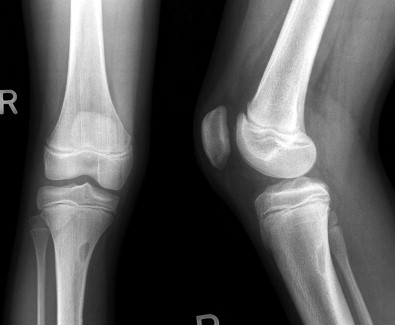
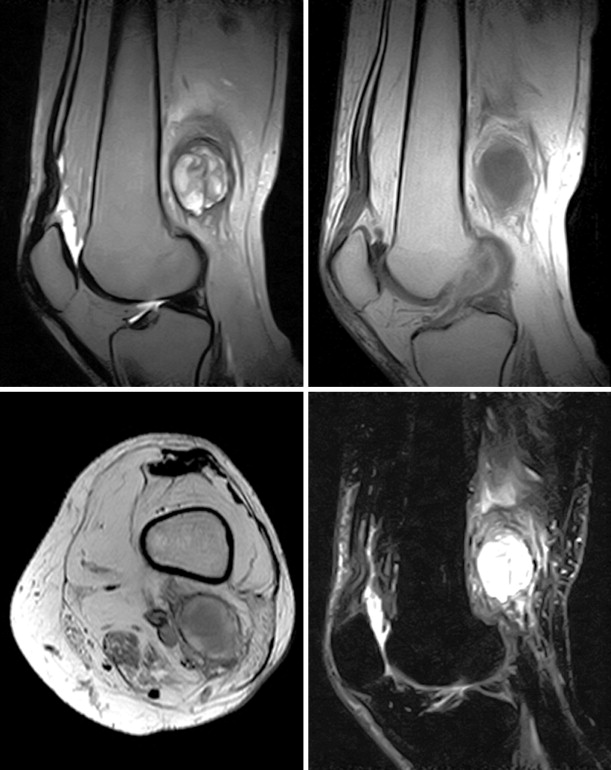
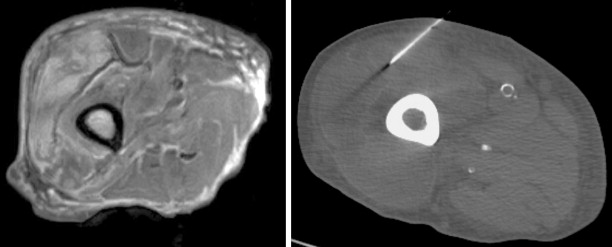
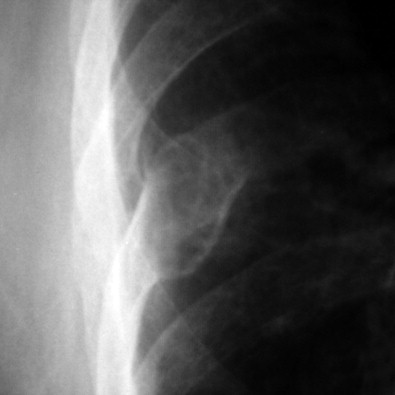
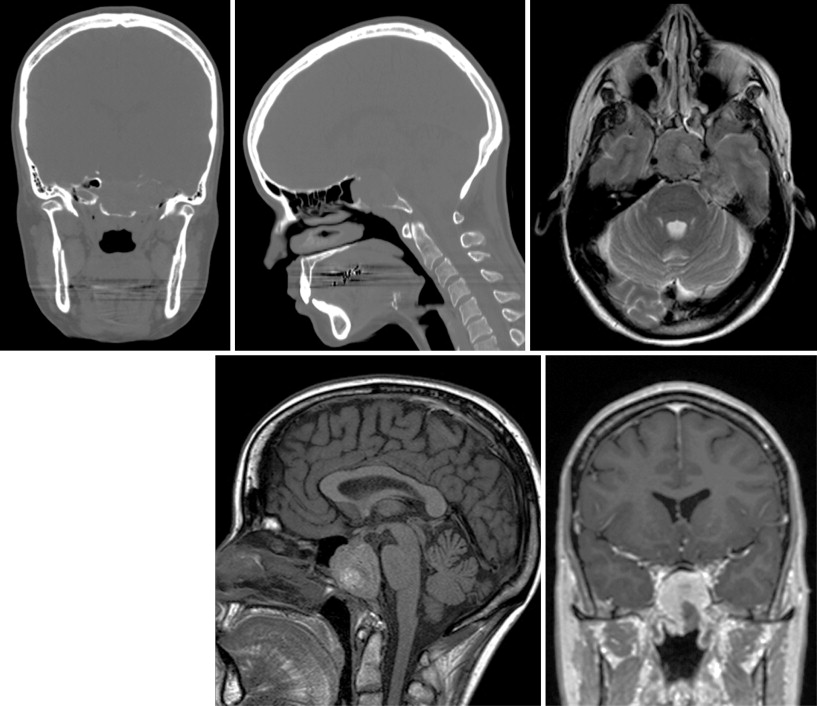
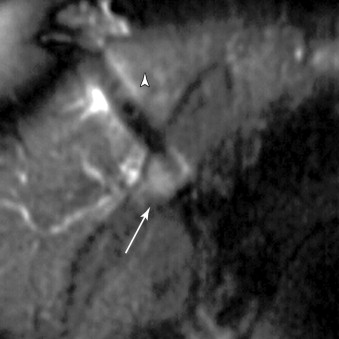
The most common reason to perform musculoskeletal biopsy is to diagnose malignancy or infection ( Table 26-1 ). Solitary bone lesions that are not clearly benign should be regarded as sarcomas until proven otherwise. There are no absolute contraindications to percutaneous biopsy ( Table 26-2 ); however, not all lesions identified by imaging warrant biopsy. A plain-film radiograph or CT of a classic osteoid osteoma does not require biopsy prior to percutaneous ablative therapy ( Figure 26-1 ). Lesions identified incidentally and believed to be latent or active but nonprogressive may be observed, for example, nonossifying fibroma or enchondroma ( Figure 26-2 ). Familiarity with common pseudotumors is essential to avoid unnecessary and possibly hazardous interventions. Lesions that can mimic a tumor include those related to soft tissues and bony structures. Trauma, overuse, infection and symptomatic normal variants are the primary causes of pseudotumors. Many magnetic resonance imaging (MRI)-detected abnormalities and mass-like lesions may have characteristic appearances on plain-film radiography or computed tomography (CT) that enable diagnosis. Examples of pseudotumors include the peripheral calcific rim of myositis ossificans, retained radiopaque foreign bodies, stress fracture of bone, and hydroxyapatite deposition within tendons. In myositis ossificans, biopsy during the early phase of development can give a false-positive diagnosis of osteosarcoma because of the intense osteoblastic activity. The diagnosis may be evident some weeks later when a peripheral rim of calcification might be seen on radiographs or CT imaging. Soft tissue hematomas may be associated with trauma or coagulation disorders, for example, hemophilia or anticoagulant therapy ( Figure 26-3 ). Complex MRI findings can result from repeated hemorrhage into the soft tissues confounded by muscle movement and time elapsed since the bleeding event. A simple hematoma can sometimes be difficult to distinguish from hemorrhage into a tumor. Diabetic myonecrosis is a myopathy associated with poorly controlled diabetes mellitus that may be associated with an intramuscular soft tissue mass ( Figure 26-4 ). Normal variants and their overuse can lead to symptoms and the appearance of a mass on MRI, for example, supernumerary bones, tarsal coalition, and accessory soleus muscle. A ruptured Baker’s cyst may present as a mass in the medial calf on MRI. Extension to the posteromedial knee should enable a diagnosis to be made. Similarly, bursitis tends to occur at reproducible locations around joints and can present as a mass. Metabolic disorders including amyloid deposition and hyperparathyroidism can also be associated with MRI pseudotumors. Brown tumors are a feature of secondary hyperparathyroidism and are frequently nonspecific on MRI ( Figure 26-5 ). The findings may be correlated with hypercalcemia, hypophosphatemia, and high serum parathormone level.
|
|
|
|
|
|
|
|
|
|
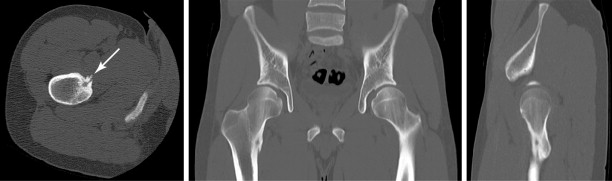
The biopsy of certain lesions needs to be approached with caution ( Figure 26-6 ). Hypervascular masses may be associated with significant morbidity particularly if they are located within a confined space, for example, a limb or the spinal canal. Hemorrhage into a limb compartment may predispose to compartment syndrome. Hemorrhage within the spinal canal may result in cord or nerve root compression. The proximity of vessels and nerves in the neck increases the complication risk for cervical spine biopsy compared with thoracic and lumbar spine biopsies. A safer route for biopsy of lesions involving the odontoid or the dens may be a transpharyngeal approach. Bone biopsy should be avoided whenever possible in the presence of local soft tissue infection.
Patient selection and procedure planning
Preprocedure planning includes a review of the patient’s clinical history, physical examination, and a thorough evaluation of all available imaging studies ( Figure 26-7 ). With a differential diagnosis in mind, the radiologist must determine whether a diagnosis can be made with the information available, confirmed by less invasive means, or that a biopsy is indeed indicated. The technical feasibility of a percutaneous biopsy is determined from a focused clinical examination and review of cross-sectional imaging studies. Planning of the percutaneous biopsy and subsequent surgical resection should then be performed in tandem, particularly if primary malignancy is a consideration and curative surgical intervention a possibility. The local extent of the lesion including proximity to the skin, neurovascular structures, joint capsule, and muscle invasion should be documented. Proximity to scar tissue and graft material from previous surgical procedures should be noted. An approach in which the risk of tumor dissemination is minimized or where the track can be resected with the lesion should be identified. , The needle path should be confined to the anatomic compartment involved, avoid neurovascular bundles, and should not cross joint spaces, bursae, tendon, or ligamentous insertions. This is particularly important for limb salvage surgery. Local recurrence rates for sarcomas after open biopsy has been shown to increase from 7% to 38% when the biopsy tract is not excised with the surgical specimen. In patients with Ewing sarcoma, local recurrence is associated with a 5-year overall survival of only 29%. Mankin et al. reported a compromised surgical procedure in 10% and unnecessary amputation in 5% of cases following a poorly planned surgical biopsy. When multiple lesions exist, the biopsy should be taken from the largest and/or most accessible lesion. Maximum yield of viable tissue may be obtained by targeting areas of increased metabolic activity seen on PET CT studies or regions that demonstrate contrast enhancement on CT and MRI scans.
The patient should be informed and educated about the procedure, its benefits, and risks. If pleural transgression is a possibility, for example, paraspinal biopsy, written informed consent should include the possibility of chest-tube placement. A history of anticoagulation therapy and antiplatelet agents should be obtained. Antiplatelet agents should be withheld 5 days before a procedure. Patients receiving low-molecular-weight heparin should have one dose held prior to the procedure. Commonly used laboratory criteria for biopsy include a platelet count greater than 50,000/µL and an internationalized normalized ratio of less than 1.5. Platelet transfusion is recommended for patients with a platelet count less than 50,000/µL. Patient comfort and cooperation may be optimized with the use of conscious sedation, including intravenous midazolam and fentanyl citrate administered by dedicated nursing staff with continuous pulse oximetry and noninvasive blood pressure monitoring. Respiratory compromise, decompensated cardiac failure, morbid obesity and diagnosed sleep apnea, pregnancy, pediatric patient status, and an inability to lie in the required position for the procedure are among the reasons that a referral to anesthesia may be required. Patient cooperation may be required for lesions close to neurologic structures in order to elicit symptoms of nerve impingement—in these cases, local anesthesia alone may be indicated. If infection is a consideration, the radiologic findings should be correlated with the white cell count, C-reactive protein level, and erythrocyte sedimentation rate. Antibiotic therapy, if already started, should be stopped for 24 to 48 hours before biopsy in order to optimize the yield of the microorganism to be sent for culture.
Technique
Imaging modality
PMB can be performed with one of a number of imaging modalities: conventional fluoroscopy, CT and/or CT fluoroscopy, ultrasound, or MRI. Ideally the biopsy should be performed in the modality in which the lesion is best visualized. Other modalities may be used as an indirect guide, for example, a lesion to be biopsied using CT or CT fluoroscopy may require correlation with MRI to identify and avoid the adjacent neurovascular structures, or ultrasound to enable tissue sampling from the solid component of the lesion. Other factors that may influence the choice of imaging used include the location of the lesion, operator expertise, and the availability of imaging modalities ( Table 26-3 ).
|
CT and CT fluoroscopy
CT and CT Fluoroscopy are the modalities most frequently used for PMB. CT provides high spatial and contrast resolution and excellent delineation of intervening structures, such as vessels and bowel, thus enabling accurate needle localization. Cortical bone destruction, bone erosion, and calcified tumor matrix are best visualized with CT. The operator can easily correlate the findings on CT and functional imaging (e.g., positron emission tomography [PET]) to target the appropriate part of the mass for biopsy and to avoid areas of necrosis. Standard CT acquisition parameters for musculoskeletal biopsies are 3–5-mm-thick contiguous transverse sections. CT fluoroscopy combines the advantages of the high-resolution imaging of CT with the real-time imaging of fluoroscopy. When using this modality, the operator should be aware of the available techniques to reduce radiation exposure for both the operator and the patient, including dedicated needle holders to keep the operator’s hand away from the gantry, using a low tube potential and current, and using CT fluoroscopy intermittently during needle advancement instead of continuously. Angulation of the gantry and/or the use of stereotactic needle guide devices may be useful when an oblique needle trajectory is required.
Fluoroscopy
Conventional fluoroscopy is widely available, is inexpensive, and enables real-time visualization of the needle during placement and tissue sampling. It delivers a lower radiation dose than CT for the same duration of exposure. It is best used for large, superficial lesions and/or appendicular lesions that are visible radiographically. Although lumbar vertebral lesions may be safely biopsied using fluoroscopic guidance, the margin for error is considerably less with thoracic and cervical spine biopsy, in which case CT guidance is advised. The low-contrast resolution of fluoroscopy limits its use in the setting of complex bone lesions.
Ultrasonography
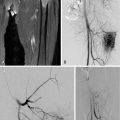
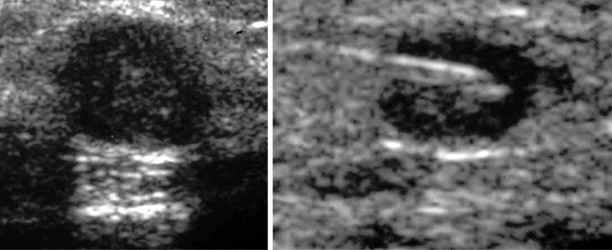
Ultrasonography may be used to biopsy a range of relatively superficial soft-tissue lesions and guide bone biopsy if cortical destruction is present ( Figure 26-8 ). Necrotic or cystic components of a lesion can be aspirated and samples sent for cytology and culture. Application of transducer pressure can displace and compress overlying fat, thus reducing the needle-path distance. In cervical spine biopsies, real-time imaging of the needle tip, the ability of color Doppler to delineate major vascular structures in and around the target lesion, and the capacity to identify alternative, oblique access routes to the target lesion reduce the risk of injury to vital structures in the neck. , Other advantages include lack of exposure to ionizing radiation, decreased procedure time, and lower cost. In the thoracic and lumbar spine regions, ultrasound guided biopsy is usually confined to posterior element lesions. A sonographic-guided anterior approach is limited by pulmonary air in the thorax and bowel gas in the abdomen that obscures the view of the lesion and overlying critical structures. The major drawback of ultrasound-guided biopsy is the fact that it is an operator-dependent method and thus may have low reproducibility. For those less experienced in ultrasound-guided interventions, a needle guide can facilitate visualization of the needle and reduce the time spent searching for the needle tip. This technique also ensures that the sampling is limited to the lesion. Lesion interpretation and the ability to target viable tumor is central to attaining high-quality diagnostic tissue samples. This is particularly challenging when dealing with heterogeneous soft tissue sarcomas. It is recommended that a standardized prebiopsy ultrasonography be performed including color Doppler and spectral wave analysis. Preliminary studies using contrast-enhanced ultrasound to delineate tumor vascularity and identify the representative areas for sampling may improve sonographic targeting of inhomogeneous soft tissue sarcomas. ,
Magnetic resonance imaging
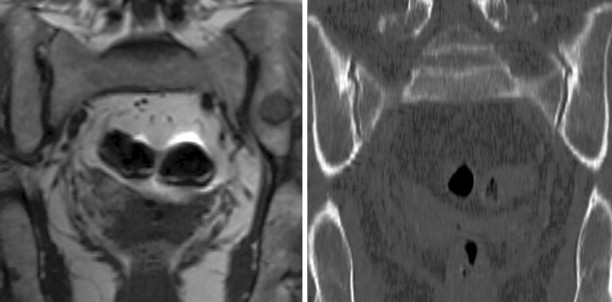
The superb soft tissue contrast resolution achieved with MRI makes it a useful tool for localizing, staging, and sampling musculoskeletal tumors. MRI provides superior delineation of the internal composition of a lesion compared with noncontrast CT and thus can facilitate targeted needle biopsy of complex inhomogeneous sarcomas. Fluid-sensitive sequences are sensitive to marrow abnormalities that are often imperceptible using other imaging modalities ( Figure 26-9 ). , Image resolution (spatial, temporal, and contrast), and rapid image acquisition are the primary yet conflicting goals of an interventional MRI sequence. This is best achieved using true Fast Imaging with Steady State Precession (FISP) type sequences. Tissue contrast resolution can be further enhanced with breath-holds and contrast medium–enhanced sequences. Needle localization is achieved indirectly through susceptibility artifact or virtually visualized using optical tracking devices ( Figure 26-10 ). The size of the needle artifact is dependent on the strength of the magnetic field, needle position with respect to the magnetic field, needle material, the sequence used, and the frequency and phase encoding directions. MRI guided PMB is becoming more popular with the advent of new open-configuration MRI systems, , continuing development in the field of MRI-compatible instruments, and ultrafast MRI sequences that allow real-time imaging. Other advantages of MRI include its multiplanar capabilities, the lack of ionizing radiation, and its ability to visualize vessels without a contrast agent. These features make it particularly suitable for biopsy of lesions in children and the pregnant patient. Multi-planar imaging allows the radiologist to identify the safest trajectory for biopsy and maintain continuous visualization of the needle trajectory in that plane throughout the procedure. This is particularly relevant for spinal lesions or lesions adjacent to vital structures. Unfortunately, the cost of installing and maintaining an interventional MRI suite, and the cost of MR-compatible instruments has limited its availability. There are also less needle choices available for MR-guided interventions. Among the trephine needles used for osseous biopsies, the titanium needles are not as strong as the steel needles used in other modalities and thus can break or bend when used in thick sclerotic bone. MRI is contraindicated in patients with pacemakers or other ferromagnetic material adjacent to critical structures.
Biopsy techniques
The aim of percutaneous biopsy is to maximize the diagnostic tissue yield and keep complications including contamination of adjacent tissues to a minimum. PMB can be performed using single needle, tandem, or coaxial techniques. One of the challenges for PMB is in accessing the lesion. In order to traverse corticated bone or access a sclerotic lesion, drill devices and/or larger bore needles (10–14-gauge) are required. With this in mind, the coaxial technique is best suited to PMB. It involves image-guided placement of an outer guide needle in or close to the lesion. A second biopsy needle is then placed through the guide needle to obtain samples. This method enables multiple samples to be acquired without having to reaccess the lesion each time. It also reduces the risk of tumor seeding or disseminating infection along the tract. The technique can be used with all imaging modalities. The tandem technique involves placement of a small needle, for example, 20–22 gauge in the periphery of the lesion under image guidance. This needle serves as a guide for placing the biopsy needle and is also used to administer local anesthetic to the subcutaneous tissues and the periosteum. The biopsy needle is then advanced parallel to the initial needle and to the same depth. Once the correct position is achieved the smaller needle can be removed. This method requires a new puncture for each sample obtained. The single needle technique involves a single needle placed under image guidance for each pass. It is associated with an increased risk of complications, increased radiation dose, and prolonged procedure time.
Biopsy devices
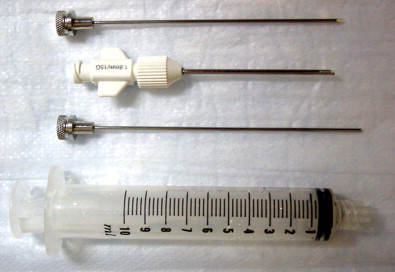
Closed biopsy of musculoskeletal lesions can be performed using fine-needle aspiration (FNA) with a small 18- to 23-gauge needle, core-needle biopsy (CNB) with a cannulated, cutting trocar system to obtain a core of tissue, or a trephine system using a stout, sharp bone-cutting tool. The needles chosen to obtain a specimen are determined by user preference, the site and appearance of the lesion, whether it is soft tissue or bone, and the volume of tissue that is likely to be required for an accurate diagnosis. The bore size of a biopsy needle is inversely related to gauge; thus, a smaller-gauge needle will yield a larger specimen. FNA and CNB are appropriate for soft tissue masses, bone tumors with a soft tissue component, or primarily lytic bone lesions. These needles cannot penetrate intact bone. Spinal needles, Chiba needles (Cook Medical, Bloomington, IN), and E-Z-EM needles (E-Z-EM, Lake Success, NY) are thin-walled 20- to 23-gauge needles with bevel angulations at the tip and are suitable for aspirating fluid, cytology of soft tissue lesions, and microbiologic sampling ( Figure 26-11 ). These smaller needles carry a lower risk of hemorrhage and tract seeding than the cutting and trephine needles. Cores of tissue may be obtained with small-bore fine needles such as the Franseen (16–25 gauge) (Cook Medical), Greene (22 gauge) (Cook Medical), Rotex (19–21 gauge) (Havel’s Inc, Cincinnati, OH), and Westcott (20–22 gauge) (Becton, Dickinson, and Company; Franklin Lakes, NJ) needles ( Figure 26-12 ). These needles have circumferentially sharpened tips to optimize the yield of cytologic aspirates and histologic core specimens. In addition, semiautomated and automated core biopsy devices (14–20 gauge), such as the Tru-Cut needle (Baxter Healthcare Corp, Deerfield, IL), Quick-core (Cook Medical), and Temno (Bauer Medical, Clearwater, Florida) can be used to obtain slices of tissue for analysis ( Figure 26-13 ). The fine needles can be inserted directly into the lesion if there is a soft tissue component or frank cortical destruction. In the majority of cases, a coaxial approach is used.
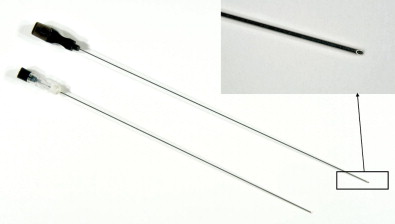
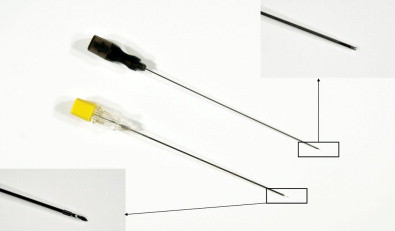
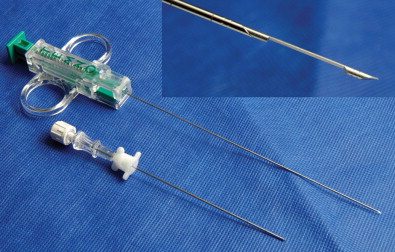
Trephine biopsy needles can penetrate intact bone and thus are the needle of choice for sclerotic bone lesions or lesions that lie deep to a thick intact cortex ( Figures 26-14 through 26-19 ). These biopsies are considerably more painful and require conscious sedation. Trephine needles may be placed using a coaxial Seldinger technique or directly. Needles placed using a Seldinger technique are used for deep bone lesions, for example, vertebral biopsy. Seldinger technique allows coaxial placement of an external biopsy cannula and trephine needle in the same track as the thin anesthetic needle using a blunt guidewire. This reduces the risk of injuring the anatomic structures with the large trephine needle. Direct needle placement is simpler and more convenient for superficial bone biopsy or biopsy in anatomic structures remote from vital structures. Most trephine systems consist of an external cannula that remains in place whereas the trephine needle is withdrawn with the sample. Some bone needles have a special cutting tip designed to penetrate hard cortical bone or sclerotic bone. The Ostycut needle (Bard) is a trephine-type needle with screw threads that allows easy advancement with a turning motion. It consists of an outer trocar that remains fixed in the lesion and allows the removal of more tissue without the need for repositioning (see Figure 26-14 ). Although the system is limited by lack of a coaxial design, a fine cutting needle can then be passed in a coaxial fashion through the trocar needle to obtain material for histopathology. The Elson/Ackerman bone biopsy needle (Cook Medical) is a reusable coaxial system. It includes an outer cannula with either a hollow or solid stylet, a 22-gauge introducer needle with removable hub (VanSonnenberg needle), and the biopsy needle with a serrated tip (see Figures 26-15 and 26-16 ).