Percutaneous Breast Biopsy
The capability of diagnosing a breast lesion percutaneously has dramatically improved the management of breast abnormalities over the last two decades. Prior to the development of these techniques, surgical excision was the procedure performed to identify the etiology of a nonpalpable mammographically detected lesion. The majority of these lesions, now classified as BIRADS 4—suspicious for malignancy—actually are benign. Until percutaneous breast biopsy was developed, many women underwent surgery for benign abnormalities to diagnose them.
Initially, fine needle aspiration biopsy (FNAB) was performed with mammographic (1) or sonographic guidance. The limitation of FNAB is the limited sampling and the need for an experienced cytopathologist for interpretation. Bolmgren (1) described the use of a stereotactic table that allowed for precise placement of a needle into a small lesion in the breast. Using this technology, percutaneous breast biopsy began its evolution. Core-needle biopsy techniques evolved as biopsy guns with Tru-cut-type automated needles of varying caliber were designed. Initially, 18-gauge core needles were used, followed by 16-gauge and subsequently 14-gauge automated core needles. The automated 14-gauge core needle is still used for the biopsy of masses, particularly with ultrasound guidance. Early results of core biopsy using a 14-gauge automated needle showed a sensitivity for malignancy of 85% (2) and a concordance between the pathologic results of core biopsy and surgery ranging from 87% to 96% (2,3,4,5). Parker et al. (3), in 1990, described biopsy utilizing stereotactic guidance in 103 patients, using a biopsy gun and automated cutting needles ranging from 18- to 14-gauge. The use of the 14-gauge needle improved the sample size and the results at pathology and, with this improvement, the management of breast abnormalities changed considerably.
Prior to the advent of the larger needles, a limited number of lesions were biopsied percutaneously—particularly cancers, complicated cysts, and fibroadenomas. However, many lesions, particularly fibrocystic lesions that were manifested as microcalcifications, yielded very nonspecific results on cytology and therefore were better diagnosed by surgical excision. With the development of percutaneous tissue sampling techniques, many more lesions could be biopsied by core biopsy instead of surgery.
The biopsy of microcalcifications has been challenging using core needles, because the volume of tissue removed is important to the accuracy of diagnosis for proliferative lesions. Vacuum-assisted breast biopsy probes have evolved to answer this problem, and larger samples can be obtained quickly and accurately using these devices. With the advent of vacuum-assisted biopsy, the speed of tissue acquisition, the volume of tissue acquired, and the accuracy for certain types of lesions have improved. Liberman et al. (6) showed that for calcifications that are highly suggestive of malignancy, the use of stereotactic 11-gauge vacuum-assisted biopsy was significantly more likely than the 14-gauge core needle or 14-gauge vacuum-assisted probe to spare a surgical procedure and was associated with the highest cost savings.
The advantages of percutaneous needle biopsy are many and include:
Improved cosmesis
Elimination of unnecessary surgery
Reduced cost
Improved efficiency in diagnosing a breast abnormality
Reduced patient morbidity
Less discomfort
Using percutaneous breast biopsy, benign lesions can be diagnosed accurately and do not require surgical excision in most cases.
Lesions that are suspicious for carcinoma can be proven to be cancer preoperatively. This is important in planning the proper surgery for the patient. Instead of performing a surgical excision for diagnosis, a lumpectomy with sentinel node biopsy can be performed with knowledge of the diagnosis. In particular, the diagnosis of invasive breast cancer on core biopsy allows for definitive therapy and increases the possibility of a single surgery. King et al. (7) reported that 90% of patients with invasive carcinoma on core biopsy could have a single surgery for definitive therapy. Liberman et al. (8) found that a single surgery was performed in 84% of women for whom the diagnosis of cancer was made on
core biopsy, in comparison with 29% of women in whom the diagnosis was made by surgical biopsy. Jackman et al. (9) found that a single surgery was performed in 90% of patients whose cancers were found on percutaneous biopsy, versus 24% of patients whose cancers were diagnosed surgically.
core biopsy, in comparison with 29% of women in whom the diagnosis was made by surgical biopsy. Jackman et al. (9) found that a single surgery was performed in 90% of patients whose cancers were found on percutaneous biopsy, versus 24% of patients whose cancers were diagnosed surgically.
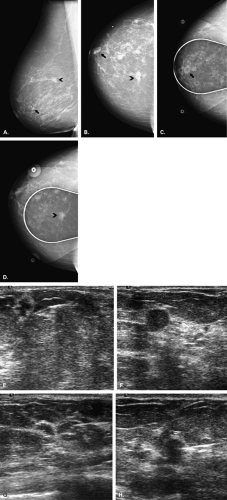
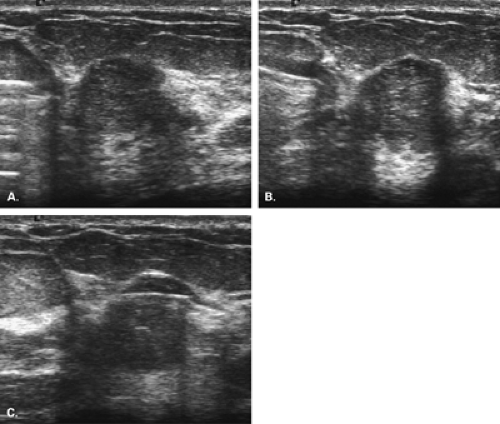
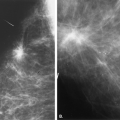
For multiple suspicious lesions, percutaneous biopsy is a very important tool to define the extent of disease. The presence of multiple cancers in one breast that involve more than one quadrant indicates multicentric disease (Fig. 16.1). This condition usually requires mastectomy for treatment. Therefore, the patient may be assessed by imaging and percutaneous biopsy, and be managed with definitive therapy—all without the need for unnecessary preliminary excisions. Rosenblatt et al. (10) found that multisite stereotactic biopsy had a positive effect on patient care in 80% of patients by helping to confirm the need for mastectomy or by documenting benign disease at multiple sites and avoiding unnecessary surgery.
Percutaneous biopsy is a less costly way of diagnosing a breast lesion than is surgery. Lee et al. (11) found an average cost savings of stereotactic biopsy versus open biopsy of $741 per case. The greatest savings occurred with indeterminate masses. Lindfors (12), in a study of the costeffectiveness of core biopsy found that the marginal cost per year of life saved by screening mammography was reduced a maximum of 23% (from $20,770 to $15,934) with the use of core biopsy instead of surgical biopsy. Liberman et al. (13) found that stereotactic biopsy decreased the cost of a breast lesion diagnosis by over 50%. Lind et al. (14) found that stereotactic core biopsy shortened the time from detection at mammography to diagnosis and breast-conserving therapy, permitted discussion with the patient of treatment options, reduced the positive margin rate and re-excision rate, and represented a cost savings in the management of nonpalpable breast cancer.
Ultrasound-Guided Biopsy
Ultrasound guidance is excellent for needle biopsy procedures in the breast. Cyst aspiration, fine-needle aspiration biopsy, and core biopsy can be performed readily for lesions that are visible sonographically. The benefits of ultrasound guidance include the ease of patient positioning, speed of the procedure, real-time visualization of the needle trajectory, low cost, lack of use of ionizing radiation, and the use of nondedicated equipment (15). Gordon et al. (16) found a sensitivity of 95% in 213 malignant lesions biopsied using ultrasound guidance and fine needle aspiration with a 20-gauge needle. Parker et al. (17) found that ultrasound-guided core biopsy using a free-hand technique with a long-throw 14-gauge core needle was a highly accurate alternative to open biopsy for the diagnosis of breast masses. Vacuum-assisted biopsy is
also performed with ultrasound guidance (18) and offers a rapid and accurate method to biopsy lesions.
also performed with ultrasound guidance (18) and offers a rapid and accurate method to biopsy lesions.
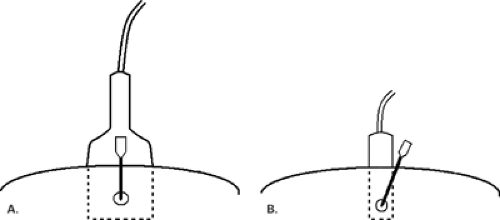
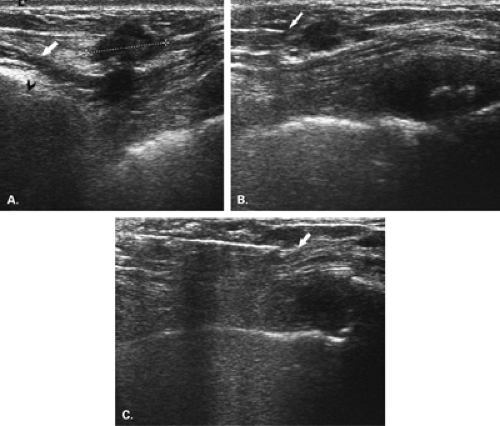
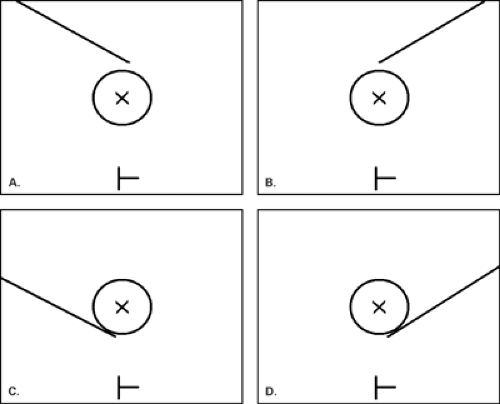
For cyst aspiration or fine-needle aspiration biopsy, a small-gauge hypodermic needle is used. The needle may be connected with thin, short tubing to a 20 cc syringe, allowing the interventionalist to handle the needle with more control. For cysts, a 1-inch long, 21- to 25-gauge needle is used. For fine-needle aspiration biopsy, the smaller the needle, the better the sample, so a 25-gauge hypodermic needle is optimal. A vertical approach, as described by Fornage (19), may be used for needle placement for cyst aspiration or FNAB (Fig. 16.2). In this manner, the needle is placed the shortest distance from and directly into the mass. For this placement, the needle is placed at the midline of the long face of the transducer, and the needle is visualized once it is at the depth of the lesion. If a long axis or horizontal approach is used, the needle is placed at the short end of the transducer and is visualized in its entirety as it is advanced toward the lesion. The horizontal approach usually requires a longer needle to reach the lesion (Fig. 16.3).
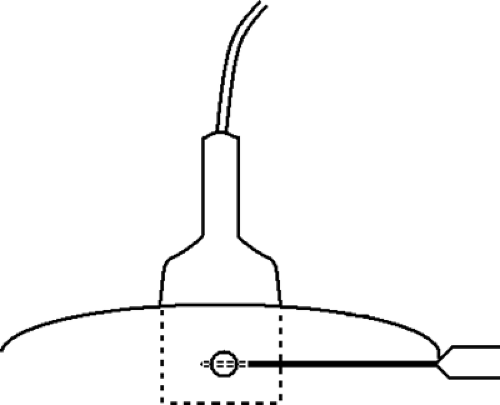
For core-needle biopsy or vacuum-aspirated biopsy, a horizontal/oblique approach must be used. Because of the forward firing of the needle, it is imperative that an approach parallel to the pectoralis major muscle be used for safety (Fig. 16.4). Many of the core needles typically used for ultrasound-guided biopsy advance into the breast about 2 cm on firing. An angled approach could easily allow the needle to penetrate the chest wall. For core biopsy, the area is cleansed with antiseptic solution, and a sterile technique is used. The device is visualized in the scan plan. It can be very helpful to have the assistant hold the transducer so that the interventionalist can use both hands to control the needle and skin and to hold the biopsy gun. Lidocaine 1% is injected for local anesthesia, and a small dermatotomy is made. The location of the dermatotomy depends on the depth of the lesion. The deeper the lesion, the farther from the edge of the transducer is the dermatotomy made. If the dermatotomy is too close to
the edge of the transducer, the needle insertion will not be parallel to the chest wall.
the edge of the transducer, the needle insertion will not be parallel to the chest wall.
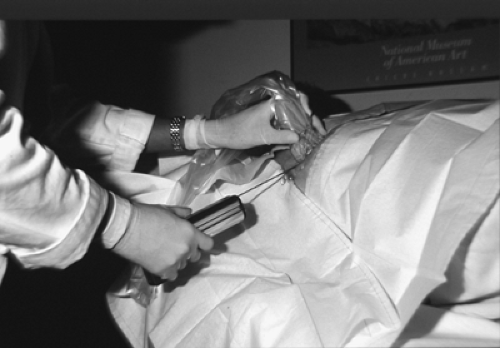
Once the needle has been placed proximal to the lesion (pre-fire position), the patient is prepared for the first sample by reminding her that she will hear a loud sound. The gun is fired and the needle traverses the lesion (post-fire position) (Fig. 16.5). Vacuum-assisted probes (VAB) may also be used for ultrasound-guided biopsy (Fig. 16.6). Using VAB, the needle may be inserted through the lesion rather than fired, and then multiple samples are acquired utilizing suction. A clip may be placed under ultrasound guidance and is particularly important for very small lesions that may no longer be clearly evident on ultrasound after the biopsy. Some of the vacuum-assisted probes are designed for clip insertion through the probe. Also, clips loaded into a stiff introducer-type needle are excellent for direct clip placement following needle biopsy.
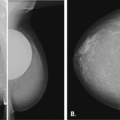
Ultrasound guided biopsy is an ideal method for the percutaneous biopsy of women with implants. The implant wall can be visualized as the procedure is performed. In the
case of patients with implants, it is imperative that care be used in administering the local anesthesia and in the dermatotomy so that the implant is not punctured. The horizontal approach must be used for needle placement and tissue sampling. Automated core needles, rather than vacuum-assisted probes, are better suited for patients with very thin breast tissue over the implant (Fig. 16.7).
case of patients with implants, it is imperative that care be used in administering the local anesthesia and in the dermatotomy so that the implant is not punctured. The horizontal approach must be used for needle placement and tissue sampling. Automated core needles, rather than vacuum-assisted probes, are better suited for patients with very thin breast tissue over the implant (Fig. 16.7).
Stereotactic Biopsy
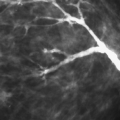
The advent of stereotaxis has greatly impacted the ease of accurate needle placement into small nonpalpable lesions and has allowed for a nonsurgical approach. Stereotactically guided needle biopsy can be performed for nodules as small as 3 mm. Stereotaxis can also be used for needle localizations and is particularly advantageous for the localization of a lesion seen clearly on only one view and superimposed over dense tissue on the orthogonal view. Stereotactic guidance with vacuum-assisted needles has become the standard or typical way of diagnosing suspicious microcalcifications.
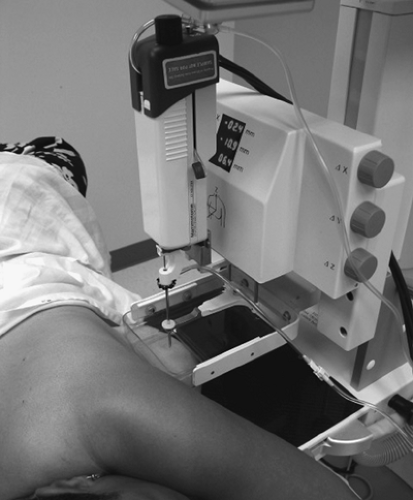
Core biopsy can be performed with stereotactic, sonographic, or magnetic resonance imaging (MRI) guidance. Stereotactic biopsy units can be prone tables on which the breast is dependant through an aperture in the table, or upright units (20) that attach to the mammography equipment. When the patient is biopsied using an upright unit, she may sit up if the lesion is located in the superior aspect of the breast. However, if the lesion can be biopsied via a medial, lateral, or inferior approach, the patient lies on a stretcher in a decubitus position for the procedure (Fig. 16.8) (21,22).
The advantages of the upright unit are the ease in positioning the patient, the access to the posterior aspect of the breast, the comfort of the patient in not having to lie prone, the ability to use the room for general mammography as well as biopsy, and the interaction of the biopsy team with the patient. Advantages of the prone table unit are the access to the inferior aspect of the breast, the lack of patient visibility of the procedure, and the rarity of a vasovagal reaction.
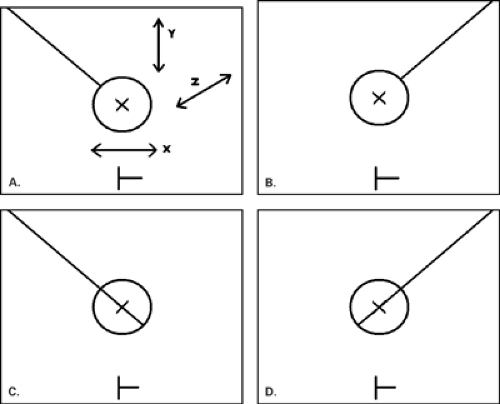
The principle of stereotaxis is based on the movement of the x-ray tube relative to the breast at angles of a fixed degree of obliquity that allow for the precise calculation of the lesion location. The technique described here is based on the Siemen’s Opdima stereotactic unit (Siemens Medical Solutions, Malvern, PA), but various stereotactic units are designed around similar basic principles and procedures. The breast is compressed and is not moved during the entire procedure. Two 15-degree oblique spot views of the lesion are made (stereotactic pair). The radiologist identifies the lesion on each image and confirms the reference point on the images. The reference point is a fixed point in the image receptor plate that is visible on the images. Based on the position of the lesion target point versus the reference point, the X, Y, and Z coordinates of the lesion are calculated by the computer. The length of needle to be used must be chosen and entered into the computer.
At the stereotactic unit, the needle is then zeroed, thereby moving the needle guides into position at a ΔX = 0, ΔY = 0, and ΔZ = 0. This position indicates that the needle tip is over the lesion on the X and Y axes and that, when the gun is fired, the target point of the lesion will be at the level of the center of the needle’s trough on the Z axis.
Following local anesthesia and a dermatotomy. the needle is placed through the guides. Repeat spot oblique views (a stereotactic pair) are made to confirm accurate needle placement. The needle position must be carefully analyzed on these pre-fire images, and these should show the needle tip at the leading edge of the lesion on both images (Fig. 16.9). If the needle is off position on the X, Y. or Z axis, its position must be adjusted based on the pre-fire images. If the needle is repositioned to any degree, a repeat stereotactic pair should be obtained. Examples of X, Y, Z, and complex errors are show in Figures 16.10, Figures 16.11, Figures 16.12, 16.13, and methods to correct the errors are described.
Once the needle position is accurate, the sampling begins. With a vacuum-assisted probe or an automated core needle, the gun is fired. The needle is fired within the breast a distance specific for each particular needle and gun; most needles advance about 2 cm into the breast. Therefore, the thickness of the breast must be sufficient to accommodate this throw or stroke of the needle. Sampling begins by retrieving tissue cores at every 2 o’clock position with a vacuum-assisted probe. Typically two rotations are made, yielding 12 core samples. This circumferential sampling pattern is rapid and accurate.
If an automated core needle is used instead, the needle is oriented in position at ΔX, ΔY, and ΔZ at 0.0 mm. The gun is fired, and the first sample is retrieved by withdrawing the needle. The needle is replaced into the breast, and similar samples are obtained at ΔX at +2 or 3 mm, ΔX at -2 to 3 mm, ΔY at +2 to 3 mm, and ΔY at -2 to 3 mm (Fig. 16.14).
For the biopsy of calcifications, specimen radiography is performed to verify that the lesion has been correctly sampled (Fig. 16.15). This step is not necessary for noncalcified lesions. The vacuum probe is withdrawn 5 mm, and a clip is placed through the probe and deployed into the middle of the biopsy site. This change in the ΔZ of 5 mm is made because the clip tends to deploy forward at the end of the trough and will lie deep to the lesion once the compression is released. A final stereotactic pair is obtained to confirm that the clip has deployed, and the needle is withdrawn from the breast. Compression is applied to achieve hemostasis, the wound is cleansed, and Steri-Strips are applied to close the dermatotomy. An ice pack is placed over the breast for 20 to 30 minutes, and the wound is assessed for hemostasis before the patient is discharged.
For aspiration procedures using stereotactic guidance, a coaxial system can be used to avoid multiple needle punctures. A 19- or 20-gauge outer cannula can be placed at the proximal edge of the lesion, and 22-gauge needles are passed through the cannula for aspiration.
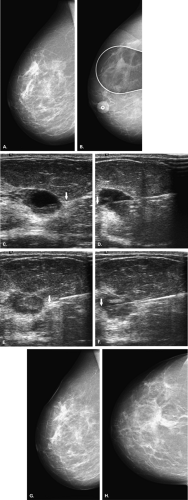
Stereotactically guided fine-needle aspiration is of help for small well-defined masses that are new in comparison with prior examinations and that are not seen or are not definitely identified as cystic on ultrasound (Fig. 16.16). Fluid can be aspirated from these cysts under stereotactic guidance. Either pneumocystography or
follow-up mediolateral and craniocaudal views should be performed to confirm the disappearance of the nodule.
follow-up mediolateral and craniocaudal views should be performed to confirm the disappearance of the nodule.
Fine-Needle Aspiration Biopsy
FNABs for palpable breast masses have been performed with success in lieu of open biopsy (23,24). For a questionably palpable lesion that cannot be satisfactorily aspirated by palpation only, or for a nonpalpable lesion, fine-needle aspiration can be performed under stereotactic or sonographic guidance. The following criteria are critical for fine-needle aspiration of nonpalpable lesions to be of value: (a) extremely accurate needle placement, (b) good techniques of aspiration and preparation of the smear, (c) experienced cytolopathologists
for interpretation of the sample, and (d) accurate and consistent results for cytologic determination of benign and malignant lesions (25). The experience of the person performing the aspiration is reflected in the percentage of aspirates that have sufficient material for analysis (23,26).
for interpretation of the sample, and (d) accurate and consistent results for cytologic determination of benign and malignant lesions (25). The experience of the person performing the aspiration is reflected in the percentage of aspirates that have sufficient material for analysis (23,26).
Regardless of the mode of FNAB guidance, a 22- to 25-gauge needle is placed into the lesion, suction is applied, and the needle is moved back and forth within the lesion. The suction is released, the needle is withdrawn, and the aspirate is ejected from the needle onto the slides and is smeared and fixed. Multiple passes will increase the yield of cells for cytologic analysis. When mammographic guidance with a fenestrated plate was used for FNAB of nonpalpable breast lesions, Helvie et al. (27) found that only 46% of the aspirates
of 215 lesions contained representative material. If an aspirate of a suspicious mammographic lesion is negative, core biopsy or open biopsy is necessary for accurate diagnosis.
of 215 lesions contained representative material. If an aspirate of a suspicious mammographic lesion is negative, core biopsy or open biopsy is necessary for accurate diagnosis.
Fine needle association biopsy under stereotactic guidance has been an option for the evaluation of nonpalpable solid lesions (28,29,30,31,32,33,34,35,36,37,38). Variable results from studies comparing cytology and histology for nonpalpable lesions have demonstrated a relatively high sensitivity and specificity for this procedure. For FNAB to be of value and to be cost effective, it is necessary that lesions called “malignant” be malignant and that no false positives occur. Otherwise, histologic confirmation would be necessary prior to definitive treatment. Because of the small size of these lesions, the mixed nature of the aspirate (38




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree