Pathology of Langerhans Cell Histiocytosis and Other Histiocytic Proliferations
Karen L. Chang
Lawrence M. Weiss
Langerhans cell histiocytosis and other dendritic and histiocytic neoplasms and related proliferations are uncommon diseases.1 Dendritic cells, also known as classical dendritic cells, arise from the myeloid-derived common dendritic cell precursor and are divided by function and structure to include Langerhans cells, interdigitating dendritic cells, and follicular dendritic cells. Langerhans cells and interdigitating dendritic cells both show strong expression of S-100 protein and HLA-DR, with Langerhans cells positive for CD1a and langerin, and interdigitating dendritic cells negative for CD1a and langerin. Normal Langerhans cells reside primarily in the skin and migrate via lymphatics (as veiled cells) to the lymph nodes as they differentiate into interdigitating dendritic cells, which reside in the paracortical region of the lymph nodes. Langerhans cells are efficient antigen-processing presentation machines, whereas interdigitating dendritic cells interact closely with T-cells, leading to the initiation of strong cellular immunity. Follicular dendritic cells express CD21, CD35, and/or CD23, and not CD1a, langerin or S-100 protein. They are fixed cells that reside in lymphoid follicles, interacting with follicular B cells in the formation and organization of the follicles. In addition, they trap and store antigen-antibody complexes and they are important in memory cell formation and affinity maturation of follicular B-cells. Plasmacytoid dendritic cells also arise from the common dendritic cell precursor and are the main source of α-interferon upon viral infection or CD154 stimulation. Plasmacytoid dendritic cells also play an important role in immune tolerance through immune regulation and/or depletion via the uptake of self-peptides captured by the uptake of apoptotic classic dendritic cells. They express CD123 and not S100, CD1a, or langerin. A subset of plasmacytoid dendritic cells has immunoglobulin rearrangements.
Histiocytes are noncirculating cells derived from a bone marrow stem cell known as monocyte/macrophage and dendritic cell precursor, which is the same stem cell that gives rise to the common dendritic cell precursor. Histiocytes are the tissue-based equivalent of monocytes and are normally resident in a variety of organs, including the spleen, lymph nodes, and bone marrow; liver (Kupffer cells); lung (alveolar macrophages); lamina propriety of the gut; and brain (microglia). They express CD163 and CD68 (an antigenic marker of lysosomes). They play an important role in resistance to intracellular microorganisms and tumors through nonimmunologic mechanisms and in the recognition and clearance of apoptotic cells.
Each of these major dendritic and histiocytic cells has corresponding tumors, as summarized in Table 61.1 and discussed in further detail below.
LANGERHANS CELL HISTIOCYTOSIS
Langerhans cell histiocytosis is a neoplastic proliferation of Langerhans cells, albeit at perhaps a more immature state of maturation.2 Langerhans cells are dendritic cells that are normally resident in the basal layer of the epidermis and are characterized by immunohistochemical expression of CD1a, S-100 protein, and langerin and the presence of Birbeck granules by ultrastructural examination. Three major syndromes of involvement are recognized.3, 4, 5 In the most common type, unifocal disease (formerly solitary eosinophilic granuloma), involvement of a single site, usually bone, is seen. This syndrome is seen primarily in adolescents and young adults. In multifocal unisystem disease (formerly many cases of Hand-Schuller-Christian disease), there is involvement of several sites within one organ system, almost always bone. This syndrome is seen primarily in children. In the least common multifocal multisystem disease (formerly many cases of Letterer-Siwe disease), multiple organs are involved, usually including the bones, skin, liver and spleen, and lymph nodes, often in a lymphoma-like distribution. These latter cases are usually seen in infants.
Langerhans cell histiocytosis represents a monoclonal proliferation, as revealed by molecular studies of the X-linked androgen receptor gene.6 There is no association with known viruses.7 Cytogenetic and other studies have shown abnormalities involving chromosomes 1p and 7.8 Recurrent mutations of the oncogene BRAF have been found in a large number of LCH cases, including the allegedly polyclonal pulmonary variant of LCH.9 Some investigators have theorized that immune dysfunction plays a role in the pathogenesis, although direct evidence is lacking. Neoplastic Langerhans cells may aberrantly express chemokines of immature dendritic cells, and abnormal reactions between Langerhans cells and macrophages may contribute to production of a cytokine “storm.”10, 11
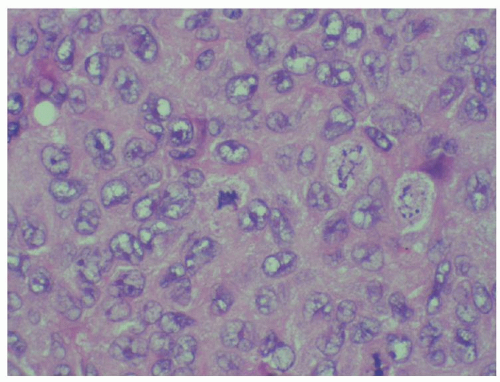
Biopsy is essential to the diagnosis of Langerhans cell histiocytosis. The key to the diagnosis is the identification of neoplastic Langerhans cells. Langerhans cells are dendritic cells with characteristic grooved, folded, or indented nuclei, inconspicuous nucleoli, a relative bland chromatin pattern, and thin nuclear membranes12 (Fig. 61.1). The degree of nuclear atypia or the mitotic rate in general does not correlate with outcome, with the exception that cases with frankly sarcomatous features probably represent rare cases of Langerhans cell sarcoma (see the section “Langerhans Cell Sarcoma”). The cytoplasm is usually moderately abundant and pale to intensely eosinophilic. Langerhans cells may be the only cell type, particularly as seen in cases of multifocal, multisystem disease, but most often they are found admixed in a characteristic cellular milieu that usually includes variable
numbers of eosinophils, histiocytes, and neutrophils. Occasionally, eosinophilic microabscesses are seen. More mature lesions have greater numbers of histiocytes, which may be nonstimulated, epithelioid, or multinucleated and simulating osteoclasts. Late lesions tend to have variable numbers of foamy macrophages and plasma cells and are often associated with a significant degree of fibrosis. Ultrastructurally, neoplastic Langerhans cells have Birbeck granules, numerous lysosomes, small vesicles, and multivesicular bodies, and irregular cell membranes with an absence of cell junctions. Birbeck granules are specific to Langerhans cells and are usually “tennis racket”-shaped intracytoplasmic membranous bodies, with an osmiophilic core and a double outer sheath. The langerin antibody is an endocytic receptor associated with the formation of Birbeck granules and thus is highly specific and sensitive and has in large part supplanted the need for electron microscopy studies.13
numbers of eosinophils, histiocytes, and neutrophils. Occasionally, eosinophilic microabscesses are seen. More mature lesions have greater numbers of histiocytes, which may be nonstimulated, epithelioid, or multinucleated and simulating osteoclasts. Late lesions tend to have variable numbers of foamy macrophages and plasma cells and are often associated with a significant degree of fibrosis. Ultrastructurally, neoplastic Langerhans cells have Birbeck granules, numerous lysosomes, small vesicles, and multivesicular bodies, and irregular cell membranes with an absence of cell junctions. Birbeck granules are specific to Langerhans cells and are usually “tennis racket”-shaped intracytoplasmic membranous bodies, with an osmiophilic core and a double outer sheath. The langerin antibody is an endocytic receptor associated with the formation of Birbeck granules and thus is highly specific and sensitive and has in large part supplanted the need for electron microscopy studies.13
TABLE 61.1 MAJOR DENDRITIC AND HISTIOCYTIC CELLS AND THEIR TUMORS | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
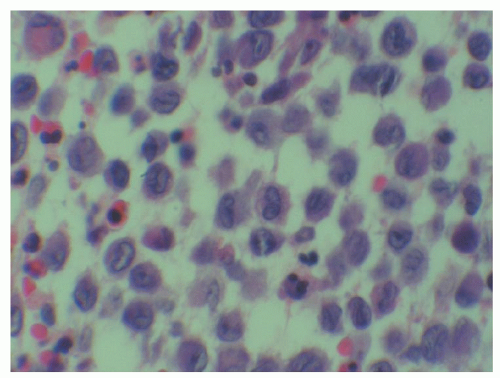 FIGURE 61.1. Langerhans cell histiocytosis. Histiocytic cells with frequent nuclear clefts are seen. Scattered eosinophils are present in the background. |
Neoplastic Langerhans cells tend to have a similar (but not identical) phenotype as normal Langerhans cells, and are usually identified by expression of CD1a, langerin, and S-100 protein14 (Fig. 61.2). In addition, Langerhans cell histiocytosis expresses vimentin, HLA-DR, placental alkaline phosphatase (in contrast to normal Langerhans cells), and peanut agglutinin lectin. The tumor has been reported to be variably positive for CD45, CD68, CD163, CD4, and lysozyme; however, expression of these antigens probably represents positivity of the intimately admixed histiocytes. The Ki-67 index is usually low (<25%). Neoplastic and normal Langerhans cells are consistently negative for B- and T-lineage markers, CD30, CD34, and follicular dendritic cell markers CD21 and CD35. By enzyme histochemistry, normal and neoplastic Langerhans cells are positive for adenosine triphosphatase, α-D-mannosidase, α-naphthyl acetate esterase, α-naphthyl butyrate esterase, and acid phosphatase.15 There is no evidence of antigen-receptor gene rearrangements.
The differential diagnosis includes reactive proliferations of Langerhans cells, including dermatopathic lymphadenitis, socalled pulmonary Langerhans cell histiocytosis, and Langerhans cell histiocytosis-like proliferations associated with malignancy lymphoma. In dermatopathic lymphadenitis, the reactive Langerhans cells occur in the paracortex, in contrast to the sinusoidal or patchy pattern of lymph node involvement by Langerhans cell histiocytosis, and the proliferating Langerhans cells are accompanied by melanin-containing histiocytes and numerous interdigitating dendritic cells. So-called pulmonary Langerhans cell histiocytosis and Langerhans cell histiocytosislike proliferations associated with malignant lymphoma are morphologically indistinguishable from Langerhans cell histiocytosis. Langerhans cell histiocytosis can be distinguished from most other histiocytic and dendritic disorders by their characteristic expression of CD1a, S-100 protein, and langerin.
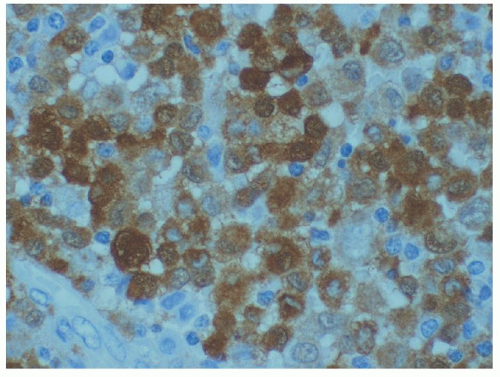 FIGURE 61.2. Langerhans cell histiocytosis, S-100 protein stain (same case as Fig. 61.1). Nuclear staining of the Langerhans cells is seen. |
LANGERHANS CELL SARCOMA
Langerhans cell sarcoma is an extremely rare neoplasm defined as a neoplastic proliferation of Langerhans cells that have overtly malignant cytologic features.16, 17, 18 It usually presents de novo but may also represent progression from typical Langerhans cell histiocytosis. There is a wide age range affected, and there may be a female predominance. Typically, multifocal multisystem disease is present. An aggressive behavior is seen, with an overall survival of ˜50%.
Histologically, a proliferation of cytologically malignant histiocyte-like cells is seen (Fig. 61.3). The presence of occasional nuclear features characteristic of Langerhans cells may suggest the diagnosis. The mitotic rate is high, and the characteristic cellular milieu of Langerhans cell histiocytosis is not usually seen. Given this histologic picture, the diagnosis is usually established with the identification of the CD1a (which may be focal; (Fig. 61.4) and S-100 protein, reactivities that are characteristic of Langerhans cells. The identification of Birbeck granules on ultrastructural examination is also helpful in confirming the diagnosis. None of the cases reported to date have included langerin study. The Ki-67 index is usually much higher than that seen in typical Langerhans cell histiocytosis.
FOLLICULAR DENDRITIC CELL SARCOMA
Follicular dendritic cell sarcoma is a neoplasm of cells differentiating toward follicular dendritic cells. Normal follicular dendritic cells are the antigen-processing cells of the germinal center. Follicular dendritic cell sarcoma is a very rare neoplasm, occurring in a wide age range and even gender distribution.17, 19, 20, 21, 22 A subset of cases may occur in association with Castleman disease, and there may also be an association with schizophrenia.17, 22, 23 Most cases are not associated with known viruses, although a subset of cases, particularly those arising in liver or spleen, may be associated with Epstein-Barr virus (EBV).24, 25, 26 It most often presents in
lymph nodes, but may also present in a variety of extranodal sites. Patients usually present with a slow-growing mass. Treatment usually consists of complete surgical excision, with or without adjuvant radiotherapy and/or chemotherapy. The tumor usually behaves in an indolent fashion, although local recurrence may occur in about one half of cases, and metastasis may occur in one fourth of cases.27
lymph nodes, but may also present in a variety of extranodal sites. Patients usually present with a slow-growing mass. Treatment usually consists of complete surgical excision, with or without adjuvant radiotherapy and/or chemotherapy. The tumor usually behaves in an indolent fashion, although local recurrence may occur in about one half of cases, and metastasis may occur in one fourth of cases.27
Histologically, one sees a spindle cell neoplasm, which may resemble a low-grade sarcoma (Fig. 61.5). The spindled cells may form fascicles and whorls, and there is characteristically an admixture of small mature lymphocytes, both amid the spindled cells or architecturally segregated into a second component, which may include secondary lymphoid follicles. The individual cells usually have bland spindled nuclei, although significant atypia may be seen in a minority of cases. The chromatin may vary from vesicular to granular and generally have small nucleoli. Occasional multinucleated cells may be seen. Ultrastructural studies show complex interdigitating cell processes with scattered mature desmosomes, typical of normal follicular dendritic cells, and an absence of Birbeck granules.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree