Pathologic Assessment of Treatment Response after Neoadjuvant Therapy
Sunati Sahoo
Susan Carole Lester
INTRODUCTION
Neoadjuvant therapy (NAT) reveals the response of cancers to therapy in vivo and, thus, generates a wealth of information for individual patients as well as for clinical trials. Pathologic downstaging after treatment is a strong predictor of disease-free and overall survival. In addition, pre- and post-therapy tumor samples are an essential resource underpinning research to understand the biologic mechanisms of how and why tumors respond. Clinical and radiologic examinations are helpful to evaluate changes in tumors during treatment, but these modalities frequently overestimate or underestimate the amount of residual carcinoma present. For example, extensive but paucicellular carcinomas may not be palpable or detectable by imaging. In contrast, densely fibrotic tumor beds often mimic residual carcinoma. Only careful pathologic examination can determine the type and extent of residual tumor and provide detailed information about treatment-related changes. However, without coordination and communication this information can be lost. In a recent review of a NAT trial for which there were no consensus guidelines for processing and reporting the specimens, only 45% of pathology reports for patients without a pathologic complete response (pCR) included an evaluation of treatment-related changes in the breast and fewer than 10% utilized a specific method to classify the degree of response (1). In addition, less than one-third of reports commented on nodal-related treatment changes. Thus, careful presurgical planning and close communication among surgeons, radiologists, medical oncologists, and pathologists are necessary to maximize the amount of pathologic information obtained from specimens after NAT.
PATHOLOGIC EVALUATION PRIOR TO TREATMENT
Breast Core Needle Biopsy
For patients who achieve a pCR, the diagnostic core needle biopsy may be the only sample of their carcinoma. Therefore, a definitive diagnosis of invasive carcinoma must be established and it is essential that all marker studies (in general, estrogen receptor [ER], progesterone receptor [PgR], and HER2) be completed before treatment. If very limited tumor is available for evaluation (e.g., < 0.5 cm) and marker studies are negative, repeat biopsies may be considered to obtain a more representative sample.
The type of breast cancer most likely to respond to chemotherapy is negative for hormone receptors, is poorly differentiated, and has a high proliferative rate. Typical pCR rates range from 30% to 40% (2). If the carcinoma also overexpresses HER2, HER2 targeted therapy often results in even higher rates of pCR. The type of breast cancer least likely to respond to therapy is well differentiated, expresses hormone receptors, and has a low proliferative rate. Lobular
carcinomas are frequently of this type. Fewer than 10% of these cancers undergo a pCR after chemotherapy (2). For these cancers, pCR is not a good predictor of prognosis, as many patients with extensive residual cancer experience long-term survival with endocrine therapy.
carcinomas are frequently of this type. Fewer than 10% of these cancers undergo a pCR after chemotherapy (2). For these cancers, pCR is not a good predictor of prognosis, as many patients with extensive residual cancer experience long-term survival with endocrine therapy.
Additional predictors of a pCR are extensive tumor necrosis and a dense lymphocytic infiltrate (3, 4). Immunohistochemical markers for identification of carcinomas more likely to be of basal type as defined by gene expression profiling (basal cytokeratins or epidermal growth factor receptor) have not been shown to predict response (5).
It is essential that a clip or clips be placed at the time of core needle biopsy to mark the site of the carcinoma prior to treatment, as this may be the only method to identify the tumor bed in many cases after treatment. If the tumor bed cannot be identified with certainty, a pCR cannot be confirmed.
Lymph Node Evaluation
The method of evaluating lymph nodes prior to treatment will impact the ability to identify node-negative and node-positive patient groups, to evaluate response in the lymph nodes, and to utilize some of the systems for classifying response to NAT.
Clinical Evaluation
Determination of nodal status by palpation alone is not accurate in many cases. In one study, 85% of patients with locally advanced breast cancer determined to be clinically node negative were found to be node positive after sentinel lymph node biopsy (6). Therefore, pretreatment nodal staging based on clinical examination alone will preclude accurate classification after treatment of cancers that never metastasized from cancers with metastases that have completely responded to treatment. This could confound efforts into investigating the biologic basis of tumor response to therapy.
Fine-Needle Aspiration (FNA) or Core Needle Biopsy of Nodes
Palpable nodes can be sampled using either FNA or core needle biopsy. If no nodes are palpable, ultrasound is a useful method to identify and biopsy suspicious nodes. Needle biopsy can confirm node positivity and also allows the evaluation of response to therapy, thus maximizing the amount of information gained from NAT.
Sentinel Lymph Node Biopsy (SLNB)
If the results of FNA or core needle biopsy do not reveal metastatic carcinoma, SLNB may be used to document node negativity before treatment. A negative SLNB prior to treatment may be more predictive of the status of the remaining nodes than SLNB after treatment due to the observation that metastases may not all respond to treatment to the same extent.
Excising a positive lymph node prior to treatment precludes the ability to evaluate response in the metastasis and also precludes documentation of a pCR in the nodes. Therefore, needle biopsies that do not completely remove the metastasis are advantageous when NAT is planned.
PATHOLOGIC EVALUATION AFTER TREATMENT
Gross Evaluation of the Breast Specimen (Partial or Total Mastectomy)
If a clinically palpable tumor mass is present after treatment (usually associated with a minimal to moderate response), the pathologist should be able to identify the mass by gross examination. However, carcinomas typically become softer and more compressible after treatment, and thus are more difficult to palpate. The reasons for the change in palpability most likely relate to loss of cellularity and the composition of the desmoplastic stroma. In addition, a decrease in blood flow is frequently detected by MRI and could contribute to a change in the firmness of the carcinoma. If the carcinoma is no longer palpable or visible on follow-up imaging studies after treatment, then the prior tumor area (tumor bed) will likely be difficult to identify. In those cases, it is preferable to have the specimen radiographed before slicing to identify a clip, calcifications, or any other radiologic finding that would localize the prior tumor site. Clips can be dislodged or lost if the specimen is sliced first. If no radiologic findings mark the tumor bed, the surgeon should place a suture at the pretreatment site of the carcinoma. If the specimen is a mastectomy, it is helpful to also provide a clock location and the distance from the nipple.
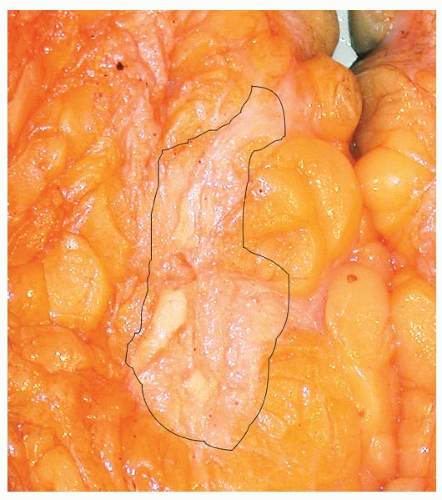
The gross appearance of a tumor bed after a marked response to NAT can be quite subtle and may consist of only an ill-defined area of fibrotic tissue (Fig. 56-1). Residual cancer may not be visible (due to low cellularity) or may consist of multiple small tan foci scattered throughout the fibrotic tumor bed.
The pathologist requires information about the number, size, and location of carcinomas prior to treatment in order to determine the best method to sample the specimen. At least one section per centimeter of the pretreatment carcinoma size of the tumor bed is suggested (7). If this sampling does not reveal residual invasive carcinoma, additional sampling may be considered to confirm a pCR.
Involvement of chest wall (skeletal muscle) or skin prior to treatment should be evaluated by sampling the areas of prior tumor involvement. These areas should be marked by the surgeon. Patients with inflammatory carcinoma should have additional areas of skin sampled. However, clinically evident skin changes often resolve during treatment and dermal lymphovascular invasion is frequently no longer seen.
Failure to find and adequately sample the tumor bed can result in an erroneous conclusion that there has been a pCR. Inaccurate information about tumor response may adversely affect patient care, evaluation of clinical trials, and interpretation of research results. If no residual cancer is present, the pathology report should clearly document the gross and microscopic identification of the tumor bed.
Gross Evaluation of the Lymph Node Specimen
The examination of lymph node specimens after treatment is the same as for specimens in the absence of treatment. All lymph nodes are identified grossly and separately evaluated by placing in designated cassettes and/or by inking nodes in different colors. Some studies report slightly fewer nodes after NAT, although other studies report similar numbers. Nodes may be more difficult to identify after NAT due to atrophy and fibrosis as a result of toxic effects of chemotherapy on normal lymphocytes. This effect could vary for different types of chemotherapy.
The nodes are thinly sliced (0.2 to 0.3 cm) and all slices are processed for microscopic examination. The use of additional levels through the paraffin blocks or immunohistochemical studies have not been shown to add useful prognostic information if the nodes appear negative on the initial H&E section. However, if scattered atypical cells are present, immunohistochemical stains for cytokeratin may be useful to identify the cells as residual carcinoma.
MICROSCOPIC EVALUATION OF BREAST CANCERS
All pathologic prognostic factors significant for untreated carcinomas are also important for carcinomas that have undergone treatment prior to surgery. However, many of these factors are more difficult to assess after neoadjuvant therapy (7, 8). In addition, the changes induced by the treatment also have prognostic importance. Although NAT protocols use a wide variety of chemotherapeutic regimes, typical changes are seen in the majority of cancers with any type of treatment. In future NAT studies with new therapeutic agents, pathologists should report any new or unusual types of tumor response.
Tumor Bed
The tumor bed consists of dense hyalinized stroma with fibroelastosis, often infiltrated by foamy histiocytes, lymphocytes, and hemosiderin-laden macrophages. It does not have the appearance of normal fibrotic breast tissue. The size of the tumor bed is generally about the size of the pretreatment cancer, although moderate increase or decrease in size are reported. The tumor bed never completely disappears but could be significantly small and subtle in rare occasion, and must be identified in order to document a pCR.
Ductal Carcinoma In Situ (DCIS)
For unknown reasons, in some patients the invasive carcinoma responds to treatment, but the associated DCIS does not. Residual DCIS can mimic invasive carcinoma on MRI, as it may continue to be associated with increased enhancement.
Many definitions of pCR allow residual DCIS (9, 10). Thus, it is very important to distinguish residual invasive from in situ carcinoma. DCIS after treatment may show marked nuclear atypia and may be intermingled with normal ductal cells and histiocytes. In difficult-to-interpret lesions, immunohistochemical studies to demonstrate myoepithelial cells associated with DCIS are helpful. Pathologists always must be provided with information about prior therapy in order to avoid misinterpreting residual in situ carcinoma in a sclerotic tumor bed as invasion. In addition, it may be difficult to distinguish atypical hyperplasia with nuclear atypia due to treatment effect from residual DCIS at margins.
Size of the Invasive Carcinoma
In cases of a minor response to therapy in which the gross carcinoma remains identifiable by palpation, a narrow rim of fibrosis may be present around the edge of the tumor. These carcinomas typically remain highly cellular. With marked response, multiple small foci of invasive carcinoma, or scattered cells, are present throughout the tumor bed (Fig. 56-2). In the absence of a pCR, the entire tumor bed would need to be excised to ensure complete removal of all foci of carcinoma.
Providing a measurement of tumor size is often difficult. The size of the entire tumor bed without notation of cellularity may overestimate residual carcinoma when there has been a marked response. Alternatively, reporting only the size of the largest single focus when multiple foci are present may underestimate tumor burden. Judgment must be used in individual cases to choose the best size for AJCC T classification (11, 12 and 13). It may also be useful for the pathologist to report the number of foci and/or the number of blocks with residual carcinoma. Because size is difficult to quantify, cellularity is also a useful parameter to assess response.
Cellularity of the Invasive Carcinoma
There is often a marked decrease in cellularity of the carcinoma, even when there is not a marked decrease in size. However, carcinomas prior to treatment also vary greatly in cellularity and a change in cellularity can only be determined with certainty if the pretreatment carcinoma is available for comparison (Fig. 56-3). Tumor cellularity is used in some systems for the evaluation of response (Table 56-1; e.g., Miller-Payne, Residual Cancer Burden [RCB]) (14, 15).
Histologic Appearance and Grade of the Invasive Carcinoma
The majority of cancers do not change in appearance after treatment, except for diminished cellularity. A few cancers may appear to be of higher grade (generally due to enlarged tumor cells with pleomorphic and bizarre nuclei from treatment effect) and in rare cases the cancer may be of lower grade due to a decrease in the mitotic index. A change in grade can only be assessed by comparing the pre- and posttreatment specimens. The prognostic significance of change in grade after therapy is unknown.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree