Angela Restrepo, Angela María Tobón, Luz Elena Cano
Paracoccidioidomycosis
Paracoccidioidomycosis is a systemic, progressive, and often chronic disease that afflicts Latin American men engaged in agriculture. The primary infection occurs in the lungs, but medical consultation is usually sought because of secondary lesions occurring in mucous membranes, skin, reticuloendothelial system, adrenals, and other organs.
Description of the Pathogen
Until 2006,1 the genus Paracoccidioides was thought to consist of a single species, Paracoccidioides brasiliensis, considered the sole etiologic agent of paracoccidioidomycosis.2,3 Presently, however, molecular studies have revealed that this genus holds four distinct phylogenetic species (S1, PS2, PS3, PS4)4,5 plus a newly described monophyletic species, Paracoccidioides lutzii.6–8 Distinguishing P. lutzii is based on a distinctive genome and difference in the conidia as well as minor differences in the yeast cell. So far, P. lutzii has only been identified in central, southwestern, and northwestern Brazil.9 Species within P. brasiliensis also appear confined to particular endemic regions.10 Because the genomic differences are not apparent in clinical manifestations and diagnosis, this chapter will allude to all Paracoccidioides species as P. brasiliensis. All species are thermodimorphic fungi ascribed to the P. brasiliensis complex in the family Ajellomycetaceae, order Onygenales.11,12 Recent genomic and morphologic analyses strongly support the existence of a sexual cycle in species of the genus Paracoccidioides.13
P. brasiliensis has been repeatedly recovered from human clinical samples,2,3 tissues of the nine-banded armadillo (Dasypus novemcinctus)14 and, more rarely, from the northern naked-tailed armadillo (Cabassus centralis).15 On occasion, P. brasiliensis has also been isolated from dogs and detected in other animal species.2,16,17
This fungus encompasses two morphotypes: a mold at temperatures less than 28° C and a yeast in mammal tissues and cultures at 35° to 37° C.2,3 Both morphotypes require ample oxygen supply for their growth.2 At temperatures less than 28° C, including 4° C if in liquid substrates, the fungus grows within 3 weeks and produces white, cotton-like colonies provided with short tufts of aerial mycelia.2,3 Microscopically, only chlamydoconidia and thin septate hyphae are observed in media such as Sabouraud,2,3 but in carbohydrate-reduced media, the fungus may produce conidia (size < 5 µm),2,18 especially if grown in sandy and claylike soils rich in water.19 Conidia respond to temperature changes by germinating into hyphae at 20° to 24° C or converting into yeasts, both in vitro (36° C) and in infected mice; in the latter, conidia initiate a progressive pulmonary process ending in fibrosis and extrapulmonary dissemination.2,3,20–22
P. brasiliensis undergoes an intricate thermal transition process by switching from a mycelial saprobic form, probably in nature,23,24 to a virulent yeast form in infected mammals tissues.1–3,22 This transition involves expression of an array of virulence factors (proteolytic enzymes, melanins, fungal adherence factors, and others),2,3,25–27 all of which facilitate P. brasiliensis interaction with its accidental mammalian hosts, interactions commanded by certain genes expressed during host adaptation.1–3,22,28,29
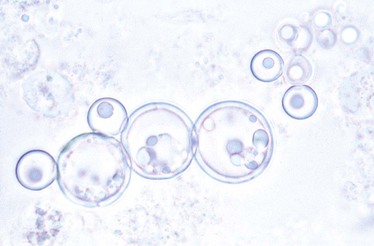
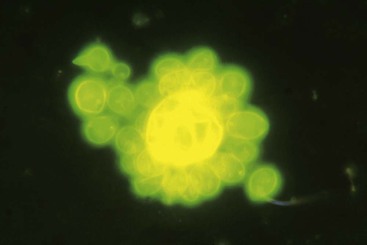
In cultures incubated at 35° to 37° C, the fungus grows in about 10 days, producing a soft, convoluted, tan-to-cream–colored colony, microscopically composed of yeast cells of varying size (4 to 40 µm), some adopting the characteristic multiple budding formation (pilot’s wheel). Isolated, round-to-oval yeast cells and short chains of blastoconidia are observed, as well as large broken yeasts (Figs. 269-1 and 269-2).2,3,22 Viable yeasts have refractile cell walls and prominent lipid vacuoles.2,3,22 Since 2009, the Broad Institute at the Massachusetts Institute of Technology (Cambridge, MA) has completed the database for the P. brasiliensis genome (www.broad.mit.edu/annotation/genome/paracoccidioidesbrasiliensis).
Ecology and Epidemiology
Paracoccidioidomycosis, a noncontagious disease, is peculiarly restricted to Latin America from Mexico (23 degrees N) to Argentina (34 degrees S), with Brazil accounting for the largest number of patients (>80%) and originating mainly from the States of São Paulo, Paraná, Rio Grande do Sul, Goiás, Rio de Janeiro, and Rondonia. Venezuela, Colombia, Ecuador, Perú, Bolivia, and Argentina follow in rank with significantly fewer cases. Uruguay, Paraguay, and the Central American countries (Nicaragua, Belize) report few or no cases. The mycosis is extremely rare in the Caribbean Islands, with only single cases having been reported from Trinidad, Grenada, and Guadalupe. The mycosis is unknown in Chile, Surinam, and Guyana.2,3,23,30–32
Areas of endemicity center around tropical and subtropical forests where mild temperatures (17° to 24° C), high annual precipitations (2000 to 2999 mm), and abundant waterways are common, conditions that favor certain agricultural crops (e.g., coffee, tobacco).2,3,23,24 Changes in traditional agricultural practices were implicated in the increased number of juvenile cases reported in Rio de Janeiro, Brazil.30 Two large Brazilian studies emphasized the connection between agriculture-related jobs and paracoccidioidomycosis, pointing toward closer or repeated contacts with P. brasiliensis in soil.31,32 Nonetheless, the fungus has been isolated only sporadically from the environment, a fact that, together with the prolonged mycosis latency periods and absence of outbreaks, has contributed to maintaining the uncertainty about the P. brasiliensis microniche.3,23 In an attempt at defining more precisely the fungus’ environmental niche, Barrozo and co-workers33,34 studied the role of weather conditions prevailing at the time of diagnosis of a number of juvenile patients, those with the shortest disease course. Absolute air humidity, soil, water storage, and presence of the Southern Oscillation Index influenced significantly the incidence, thereby suggesting their connection with acquisition of the infection.34
The disease is characterized by long periods of latency—30 or more years—as demonstrated by the nonautochthonous cases reported outside recognized endemic areas (North America, Europe, Asia).35,36 In every case, however, the patient had previously resided in an endemic country where the infection should have occurred, as exemplified by cases reported in Spain, France, and Japan.37–39,40
Imprecise knowledge on the fungal habitat has also hindered definition of the infection route, although clinical and experimental animal studies have ruled out traumatic implantation and pointed instead toward the aerosol route.2,3,20,35
In a series of 1000 Brazilian cases, incidence varied between 1.6 and 3.7 patients per 100,000 persons per year.32 In Colombia, these figures were lower and fluctuated greatly (between 0.5 and 2.2 per million persons), based on the year of report.41 In a series of almost 2000 fatal cases reported in São Paulo, Brazil, the mycosis accounted for 60% of the deaths.42 Another Brazilian study analyzed more than 3000 fatalities attributed to systemic mycoses, finding that paracoccidioidomycosis caused greater than 50% of the deaths, especially if associated with acquired immunodeficiency syndrome (AIDS).43 In the Itaipu Lake in the Brazilian Paraná State, Loth and co-workers44 reported the occurrence of 102 new patients diagnosed in a period of only 18 months, with 15 deaths. The above data have been analyzed by Martinez,45 who emphasized the importance of this mycosis in Brazil and rightly called it a neglected disease because no major health regulations have been implemented to curtail the problem.
Coinfections may develop in paracoccidioidomycosis patients, especially those with AIDS and tuberculosis. In a representative series, paracoccidioidomycosis was found in 1.4% of AIDS cases.46 As for tuberculosis, a report found active cases in 8% of the paracoccidioidomycosis patients analyzed. Misdiagnosis was common because of the similarity between the clinical and radiographic presentations.47
The age and gender distribution of clinical cases is peculiar. The mycosis is rare in children (<2%) and teenagers (<9%), with the remaining patients being 30 years of age and older.48 Several series encompassing more than 5000 patients revealed that the male-to-female ratio was 11 : 1.48 This proportion contrasts with the infection rate as determined by a reactive paracoccidioidin skin test, which had a similar prevalence for both genders.49 Of note, when the disease occurs in prepubertal patients, no gender difference becomes apparent, as found with more than 100 children in whom the boy : girl ratio was 1.1 : 1.0.48 In vitro, Paracoccidioides exhibits a strong hormonal effect in that 17–β-estradiol blocks or delays the transition from the infecting propagules (mycelium or conidium) to the yeast form.48
The occupational distribution reveals a predilection for individuals who have been engaged in agriculture (>60%) or have had soil-related jobs with subsequent exposure to infected soil and dust, mainly in connection with coffee, cotton, tobacco, and sugar cane plantations.32,35,36,50 Increased acreage of sugar cane fields in the southwestern region of Brazil may alter these data because this crop requires pesticide application and plant burning, a combination that may affect P. brasiliensis saprobiotic life in nature.35 Other occupations mentioned as relatively frequent are masonry and lumberjacking.35,36
Pathogenesis
Paracoccidioidomycosis encompasses a subclinical infection resulting from the initial contact with the fungus and an overt disease manifested later. In healthy blood donors, subclinical infection was evidenced by a reactive skin test and the presence of anti-gp43 antibodies. A more definitive demonstration of silent infection came from observing P. brasiliensis in residual lesions.4,5,14,31,32 The overt disease is always a disseminated process with two main clinical presentations—the juvenile and the chronic adult disease.27,30 The former is characterized by marked involvement of the reticuloendothelial system and occurs in less than 15% of cases; it is thought to represent progression after a rather recent exposure.25–29 The hallmarks of the chronic adult type of disease are significant lung involvement and extrapulmonary lesions. This is the predominant form occurring in approximately 90% of cases and probably represents endogenous reactivation after years of initial contact with the fungus.6,7,33 Results of gallium-67 scans have revealed multiorgan involvement in almost all the adult cases; in patients with the juvenile form, extralymphatic involvement is more clinically obvious.34 A residual form is also recognized and is represented by fibrotic scarring occurring at the sites of previously active lesions.35,36 A recent cluster analysis done in patients with concomitant lung and mucosal and/or skin lesions has strongly supported the existence of two different sets. The first analysis includes patients with mucosal damage, odynophagia, and/or dysphagia, plus alveolointerstitial infiltrates, and the second one consists of patients exhibiting dermal lesions, dyspnea, and lung fibrosis. The former conditions would represent early stages of the infection, whereas the latter would correspond to a more chronic granulomatous and fibrosing process.37
P. brasiliensis infection may become dormant but be reactivated later under the influence of ill-defined conditions prevalent in rural settings, such as chronic alcoholism, malnutrition, and smoking.*
Pathogenesis and the Immune Response
The pathogenesis of this mycosis depends partly on P. brasiliensis virulence factors and antigenic composition, on environmental conditions, and also on the efficiency of the host immune response.51 Different in vitro, in vivo, in silico, and ex vivo experiments, as well as clinical studies, have demonstrated that specific and nonspecific defense mechanisms, both innate and adaptive, are determinative in building host resistance.9 Consequently, this response is highly complex and multifactorial. If one accepts that a particular balance of the host-fungus interaction is crucial for the efficient control of infection, one could postulate that paracoccidioidomycosis would develop only in those fungus-infected individuals who have lost this balance.51 Then, it is important to point out that the initial interaction of the microorganism with the first line of host defenses would determine the type of response to be developed (protective, deleterious), and, accordingly, would determine the disease course.51
Innate Immune Response
This response begins at the level of the pulmonary alveoli, where the microorganism lodges upon host inhalation. Then, certain pulmonary epithelial cells, endowed with the capacity to produce different molecules, initiate the immune response. In vitro studies have shown that P. brasiliensis yeasts stimulate the lung’s A549 epithelial cells to produce interleukin-6 (IL-6) and IL-8 by a process dependent on activation of kinases (p38 mitogen-activated protein kinase [MAPK], extracellular signal-regulated kinase [ERK1/2]). In contrast, viable fungal cells are capable of degrading the above cytokines through protease expression, suggesting that P. brasiliensis has the capacity to modulate the host’s inflammatory immune response.52
Once in the lungs, P. brasiliensis propagules interact with lung cells and extracellular matrix proteins (ECMPs). Adhesins such as the glycoprotein gp43 (the main P. brasiliensis immunodominant antigen), other proteins (19, 30, 32 kDa), malate synthase, triosephosphate isomerase, glyceraldehyde-3-phosphate dehydrogenase, and enolase are expressed on the P. brasiliensis cell surface, where they attract molecules for the recognition of ECMPs (laminin, fibronectin, fibrinogen, plasminogen) and facilitate fungal adherence.53
Besides the lung epithelial cells, the first line of defense is represented by polymorphonuclear (PMNs) leukocytes, alveolar macrophages, dendritic cells, natural killer (NK) cells, complement, peptides, proinflammatory cytokines, and chemokines, all of which hinder fungal multiplication but are unable to destroy the invading microorganism.50,51
Innate immune system cells detect fungal presence through pattern recognition receptors (PRRs) that attach to conserved molecular structures present in the microorganisms but absent in the host, known as pathogen-associated molecular patterns (PAMPs). Among the PRRs that participate in the innate immune response against P. brasiliensis, Toll-like receptors (TLRs) and dectin-1 play important roles, as demonstrated in healthy individuals whose PMNs and monocytes had been previously stimulated with the fungus. As a result, such phagocytic cells express TLR2, TLR4, and dectin-1, thus able to recognize and internalize fungal propagules.51,54
Healthy human PMNs activated in vitro with granulocyte-macrophage colony-stimulating factor, IL-15, tumor necrosis factor-α (TNF-α), or interferon-γ (IFN-γ), and infected with yeasts from a virulent P. brasiliensis strain, revealed that chosen cytokines were able to induce an increased expression of TLR2 and TLR4. The fungus increased TLR2 expression but inhibited the corresponding TLR4.55 In addition, experimental in vivo studies using TLR2 knockout mice that had been infected with P. brasiliensis by the intratracheal route revealed that deficiency of this factor favored development of a less severe pulmonary infection compared with the control group. This treatment increased KC (a chemokine that facilitates PMN chemotaxis), transforming growth factor-β (TGF-β), IL-6, IL-23, and IL-17. This Th17 profile was associated with decreased expansion of regulatory T cells.54 In addition, the authors demonstrated that fungal lipid components modulated macrophage activity through TLR-dependent and -independent mechanisms.54
Various experimental studies have shown the presence of dense infiltration by PMNs in the lungs of animals infected with P. brasiliensis correlated with cytokine (TNF-α, IL-6, IL-1β) and chemokine (macrophage inhibitory protein 2 [MIP-2]) secretion, as well as with the expression of adherence molecules (intercellular adhesion molecule 1 [ICAM-1], vascular cell adhesion molecule 1 [VCAM-1]) and integrins, facilitating the influx of such phagocytes.56
NK cells also participate in the innate immune response against P. brasiliensis, In vitro studies demonstrated that NK cells from patients with the mycosis exhibited decreased cytotoxic response compared with those from healthy individuals. NK cells were capable of recognizing and killing extracellular yeasts through a mechanism dependent on granules and independent of perforins, whereas cytotoxicity against the intracellular fungus depended on perforin. Granulysin seemed to be a possible mediator of the granules-dependent mechanism because this molecule was detected in NK cells plus P. brasiliensis supernatants and was capable of killing the fungus in a dose-dependent manner. In addition, it has been shown that patients with the mycosis had a low frequency of CD56+ granulysin+ cells compared with healthy control subjects. Reports indicate that NK cells, once stimulated with recombinant (r)IL-15, are capable of producing proinflammatory cytokines (IFN-γ, TNF-α) that confer an immunomodulatory role in paracoccidioidomycosis.57
Macrophages represent the major cellular defense against P. brasiliensis. When activated by IFN-γ, they ingest and kill both conidia and yeasts through expression of inducible nitric oxide synthase (iNOS).51,56 Nitric oxide, however, may play a dual role (resistance and susceptibility), depending on the degree of expression.56
Fungal extracellular proteins represent key mediators in the host-fungus interaction, significantly influencing the type of response that will be developed. Modern proteomic strategies that combine two-dimensional electrophoresis with matrix-assisted laser desorption/ionization quadrupole time-of-flight mass spectrometry (MALDI-Q-TOF MS), were used to analyze the P. brasiliensis mycelium and yeast secretomes. The study identified 160 nonredundant protein isoforms, including 30 and 24 proteins preferentially secreted by the mycelium and the yeast, respectively. Addition of brefeldin A to culture media significantly reduced the production of the fungal-secreted proteins, regardless of whether they were located extracellularly or were intracellular in the macrophage. On the contrary, addition of concentrated supernatant cultures containing the secreted proteins, increased significantly the number of yeasts internalized by the macrophages.58
Thus, the host’s immune response represents the summation of a series of biologic effects resulting from fungal interaction with different cells, molecules, and receptors geared at eliminating the microorganism. If this is not achieved, the specific or acquired immune response, cellular or humoral, will then be initiated.51
Acquired Immune Response
Cell-mediated immunity is crucial to defense; it is usually depressed at the peak of the disease but can be restored with successful treatment. Suppression of the cellular immune response is characterized by negative delayed-type hypersensitivity to P. brasiliensis antigens, lymphocyte apoptosis, and high expression levels of cytotoxic T-lymphocyte–associated antigen-4 (CTLA-4), interleukin-10 (IL-10), and TGF-β.
The dichotomy between humoral and cellular immune responses suggests a helper T-cell 2 (Th2) pattern of immune response.50,51 Thus, juvenile patients and those with the chronic adult form disease have nonreactive paracoccidioidin skin tests and detectable anti–P. brasiliensis antibodies, show depressed lymphoproliferative responses to the gp43 antigen, and cytokine patterns corresponding to Th2-type immunity; for instance, low IFN-γ secretion, high levels of IL-4, IL-5, and IL-10, plus impaired IL-12 synthesis.50,51 Addition of IL-12 to patients’ macrophages and neutralization of IL-10 increased IFN-γ levels, suggesting the possibility of introducing immune modulation to restore patient defenses. In adult patients with disease of minor or intermediate severity, immune responses are strong (e.g., higher specific lymphoproliferative responses than in the juvenile patients). Thus, patients’ profiles are compatible with the presence of low and high resistance to fungal invasion. In contrast to patients with clinical manifestations, infected individuals (i.e., skin test reactive) living in the endemic area and who have no clinical manifestations of the disease exhibit an opposite, normal Th1 immune profile. Cytokine and chemokine expression kinetics appear to distinguish P. brasiliensis infection from overt disease because P. brasiliensis–infected individuals demonstrate expression of various messenger RNAs for specific cytokines or chemokines, all of which differ in quantity and timing of expression, based on the disease form.51,59
Compact granulomas are considered the most evolved and effective biologic defense weapon against P. brasiliensis.2,3,22,50,51 Granuloma formation involves the activity of T lymphocytes (helper and cytotoxic subsets); activated effector cells, mainly represented by macrophages but also by neutrophils; and several cytokines, especially IFN-γ and IL-12. Th2 cytokines (IL-4, IL-10, TGF-α, or TGF-β) are associated with increased host susceptibility, probably because they interfere with correct macrophage function.22
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree