Paget’s Disease
Nora M. Hansen
Paget’s disease, a rare presentation of breast cancer accounts for 1% to 3% of all breast cancers (1, 2). The majority of cases are associated with an underlying malignancy and prognosis is dependent upon the stage of the underlying cancer (3). In a recent review of the Surveillance, Epidemiology, and End Results (SEER) data by Chen et al. (4), it was reported that the incidence of Paget’s disease has decreased between 1988 and 2002. The age-adjusted incidence rates decreased by 49% for Paget’s associated with invasive ductal cancer and by 44% for Paget’s associated with ductal carcinoma in situ (DCIS).
The first description of Paget-like features was in 1307 by John of Arderne who recorded the several-year evolution of nipple ulceration in a male priest, with the subsequent development of a breast cancer. Velpeau is typically credited with the first clinical description of Paget’s disease when, in 1840, he described the visual surface lesion of Paget’s disease in two patients. It was in 1874 that Sir James Paget recorded the association of the clinical findings with an underlying breast cancer in 15 patients, although he speculated that the chronic skin condition was benign. It was Thin, in 1881, who concluded that the nipple lesion was not a benign entity, but a malignant one. He postulated that the nipple lesion contained cells that were related to the underlying cancer which had extended to the nipple through the major lactiferous sinuses which we refer to today as Pagetoid spread.
PATHOGENESIS
The pathogenesis of Paget’s disease is an interesting one because there are two main theories for the origin of Paget’s disease. The most widely accepted one is the epidermotropic theory, first described by Jacobeus, who suggested that the Paget cells arise in breast ducts and spread through the lactiferous sinuses to the nipple epidermis. This view is supported by several observations. First of all, it is well documented that most patients with Paget’s disease have an underlying breast carcinoma which is ductal in origin and that the immunohistochemical profile and pattern of gene expression in the Paget cell and the underlying cancer are similar (5). In addition, heregulin alpha, a motility factor released by normal epidermal keratinocytes can induce chemotaxis of the Paget cells to migrate into the overlying nipple epidermis (6).
Because not all Paget’s disease is associated with an underlying carcinoma, another theory, the intraepidermal transformation theory (in situ transformation theory) proposes that the Paget cells arise either in the terminal portion of the lactiferous duct at its junction with the epidermis or from multipotential cells in the epidermal basal layer. This in situ transformation theory is thought to occur in pre-existing benign intraepidermal clear cells of the nipple areolar complex, or Toker cells, which are thought to have migrated from nonneoplastic ducts (7). Support for this theory is found in the rare cases of Paget’s disease without an underlying breast carcinoma or cases in which the Paget’s disease and the underlying carcinoma appear to be separate tumors (8). Other studies have identified desmosomal attachments between the Paget cells and adjacent keratinocytes supporting the in situ development of the Paget cell (9).
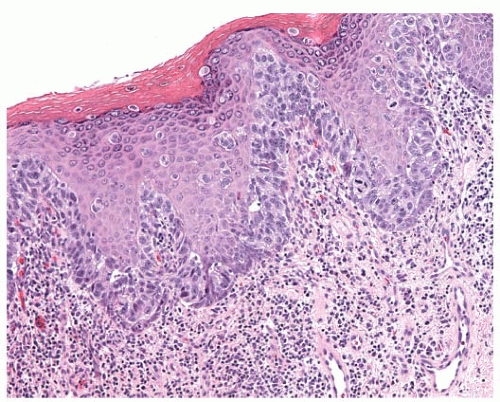
Histologically, Paget’s disease is characterized by the infiltration of the nipple epidermis by Paget cells, described as large pale-staining cells with round or oval nuclei and prominent nucleoli. The cells are between the normal keratinocytes of the nipple epidermis, occurring singly in the superficial layers and in clusters toward the basement membrane. Serous fluid can seep through the disrupted keratinocyte layer, resulting in the crusting and scaling of the nipple skin. Paget cells can traverse the epithelium and, thus, sometimes are found in the superficial layers. The basement membrane of the lactiferous sinuses is in continuity with the basement membrane of the skin. Paget cells do not invade through the dermal basement membrane and therefore are a form of carcinoma in situ. Paget’s disease is often associated with a chronic inflammatory infiltrate in the dermis (Figs. 63-1 and 63-2).
INCIDENCE
The incidence of Paget’s disease varies whether one refers to the pathologic or clinical entity. Paget’s disease is a more common pathologic than clinical entity (1, 10). Its clinical incidence ranges from 0.5% to 2.8% with a mean of 1.3% in more than 50,900 patients combined from nine studies (1, 10, 11). Histological evidence of Paget cells is present in 0.5% to 4.7% of nipples from breast cancer specimens (1). In a series by Lagios et. al. (10) of 3,000 consecutive breast cancer mastectomy specimens, 21 (0.7%) had clinical evidence of Paget’s disease and 147 (4.9%) had Paget cells histologically, thus yielding a sevenfold difference.
Of the 158,621 microscopically confirmed female and male breast cancer registrants from the SEER registry of the National Cancer Institute, 1,775 (1.1%) had histologic Paget’s disease (12). Of patients with breast cancer from this database, Paget’s disease was histologically identified in 1.1% of white female patients, 1.3% of African American female patients, 1.1% of white male patients, and no African American male patients. Clinical Paget’s disease has been reported in patients ranging in age from 23 to 90 years, with mean ranging from 53 to 65 years (13, 14, 15, 16 and 17). In a further analysis of the SEER data, the mean age of women with Paget’s disease was 62 years and that of the men was 69 years. This was not significantly different from female (61 years) and male (67 years) patients with ductal breast cancer. In an update of the SEER data, Paget’s disease associated with both invasive and DCIS has decreased from 1988 to 2002 by 49% and 44%, respectively (4). However the number of cases of Paget’s diagnosed without underlying invasive disease may be increasing. This may be accounted for the increased use of mammography and finding cancers at an earlier stage before they develop Pagetoid features.
Paget’s disease is not confined to women and can occur in men but it is extremely rare. There are probably less than 50 cases described in the world medical literature and most are case reports. There was one report of bilateral Paget’s disease of the male nipple with the patient being treated with bilateral mastectomies.
CLINICAL PRESENTATION AND DIAGNOSIS
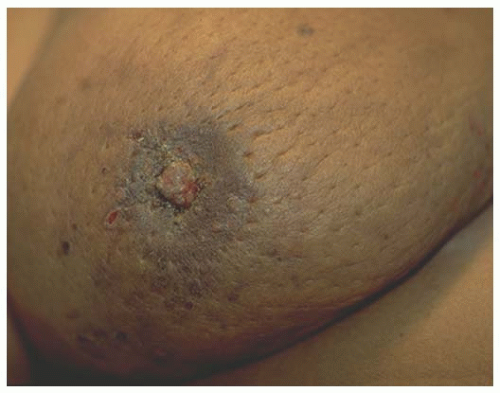
The majority of patients with Paget’s disease present with eczema or ulceration of the nipple and many have a prolonged period of symptoms before diagnosis. It is, therefore, important to have a high index of suspicion for Paget’s when a patient presents with nipple complaints. The most common initial presentation is erythema and mild eczematous scaling which progresses to crusting, skin erosion, and ulceration, with exudation or frank discharge (Figs. 63-3, 63-4 and 63-5).
The clinical differential diagnosis of scaling skin and erythema of the nipple-areola complex in addition to Paget’s includes eczema, contact dermatitis, and post radiation dermatitis. Although bilateral Paget’s has been reported, bilateral symptoms are most consistent with eczema or contact dermatitis. Skin changes that are confined to the areola and spare the nipple are typically attributed to eczema, although they can occur rarely in Paget’s disease. The clinical
differential diagnosis has prompted initial topical steroid treatment, often with transient improvement of symptoms (3). Other patients have been treated with antibiotics. In patients who have been previously treated with breast conservation, Paget’s disease may mimic post radiation scaling. Given the infrequency of Paget’s disease in this setting, the diagnosis of Paget’s disease may be delayed. Symptom duration preceding the diagnosis of Paget’s disease is variable and averages 91/2 weeks to 27 months, with a range of 1 week to 20 years (18). In a study from the MAYO clinic, the median duration of symptoms was 6 days with a range of 1 to 80 days, clearly a unique patient population (14).
differential diagnosis has prompted initial topical steroid treatment, often with transient improvement of symptoms (3). Other patients have been treated with antibiotics. In patients who have been previously treated with breast conservation, Paget’s disease may mimic post radiation scaling. Given the infrequency of Paget’s disease in this setting, the diagnosis of Paget’s disease may be delayed. Symptom duration preceding the diagnosis of Paget’s disease is variable and averages 91/2 weeks to 27 months, with a range of 1 week to 20 years (18). In a study from the MAYO clinic, the median duration of symptoms was 6 days with a range of 1 to 80 days, clearly a unique patient population (14).
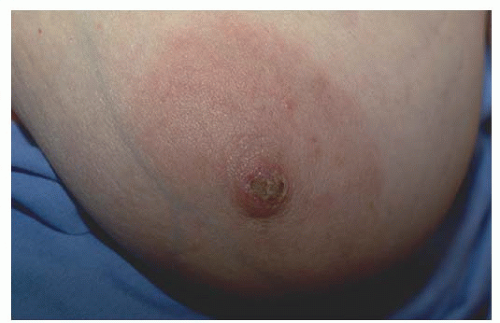 FIGURE 63-3 Paget’s nipple. Erythema and crusting of nipple occupying the majority of the nipple surface. |
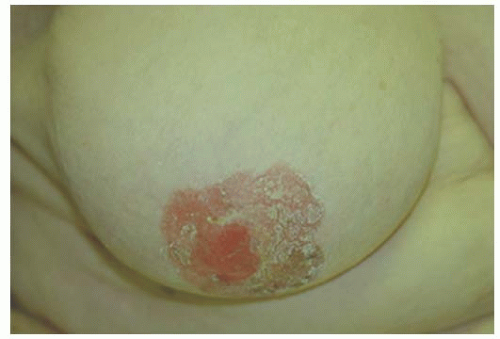 FIGURE 63-5 Paget’s nipple. Advanced Paget’s with crusting and ulceration extending from nipple and encompassing areola. |
Less common diagnoses in the clinical differential of mammary Paget’s disease include nipple adenoma, papillomatosis, melanoma, Bowen’s disease, and, rarely, basal cell carcinoma, squamous carcinoma, sebaceous carcinoma, Merkel cell carcinoma, infiltrating lobular carcinoma, cutaneous T-cell lymphoma, Spitz nevus, epidermotropic metastases, alteration of keratinocytes present in 10% of normal nipples. Toker cells can be distinguished from Paget cells by their lack of nuclear pleomorphism or cytologic atypia and their absence of mucin (19).
Although most patients with Paget’s present with nipple changes, up to 50% will present with a palpable mass. The majority of patients who present with a palpable mass have an underlying invasive cancer and thus have a worse prognosis. Patients who present with a palpable mass also have a higher rate of nodal positivity (3, 20). It has also been shown that HER2-neu overexpression has been found in both DCIS and invasive disease associated with Paget’s and this may, in part, account for the poorer prognosis associated with Paget’s.
The diagnosis can be obtained by scrape cytology, a superficial epidermal shave biopsy, a punch biopsy, a wedge incision biopsy, or nipple excision (19). The ideal specimen contains adequate epidermis to provide Paget cells and a lactiferous duct. Paget cells may be distributed in a patchy fashion throughout the nipple, so additional specimen sampling may be required to secure the diagnosis.
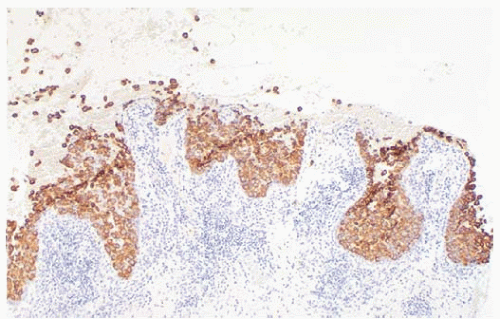
The histologic differential diagnosis of Paget’s disease includes superficial spreading melanoma, squamous cell carcinoma in situ (Bowen’s disease) and clear-cell changes of squamous cells of the epidermis (Toker cells). The cell type can be determined by immunohistochemical studies including low-molecular-weight keratins (CK7, cellular adhesion molecule 5.2[CAM-5.2]), broad-spectrum keratins, melanoma antibodies, and mucin stains (19).
Paget cells are immunoreactive for keratins (CK7, CAM-5.2, and AE1/AE3), occasionally are immunoreactive to S100 (21), and are not immunoreactive for HMB45 or high-molecular-weight keratins (22). In one study, mucin was present in 55% of 20 patients and, thus, was not informative in 45% of patients (22). Paget cells can phagocytose melanin from adjacent epidermal melanocytes and may be mistaken for melanoma if immunohistochemistry is not performed (22). Melanomas are immunoreactive for S100, are often immunoreactive for HMB45 and are only very rarely immunoreactive for low-molecular-weight (CAM-5.2), broad-spectrum keratins (AE1/AE3), or mucin stains (22). Squamous cells are immunoreactive for low-molecular-weight keratins and broad-spectrum keratins (AE1/AE3) are infrequently immunoreactive for S100 and are not immunoreactive for CAM-5.2, HMB45, or mucin stains (50). Toker cells, or clear-cell changes of the epidermal squamous cells, are a non-neoplastic DCIS, and 1% had Paget’s disease only. Toker cells are concentrated within the basal layer or arranged into glandular structures growing up to the spinous layer. These clear cells of the nipple were first described by Cyril Toker in 1970 (7). On occasion these cells can be numerous and atypical and can be difficult to distinguish from malignant cells of Paget’s disease. In a study by Di Tommaso et al. (7), Toker cells were identified in 10.2% of patients who underwent mastectomy. In the majority of cases the Toker cells were cytologically bland and benign, while in 27.5% of cases the Toker cells were more numerous and persistent on serial sections and referred to as hyperplastic Toker cells. In 12.5% of cases the Toker cells were hyperplastic and atypical. On immunohistochemistry Toker cells were positive for estrogen receptor and progesterone receptor and negative for CD138 and p53. In comparison Paget’s cells were negative for ER and PR and positive for HER2/neu. Both Toker and Paget’s cells stained positive for cytokeratin 7 and epithelial membrane antigen and negative for p63 (7).
RADIOGRAPHIC EVALUATION
In patients with clinical Paget’s disease, the reported incidence of mammographic findings varies in the literature. For those patients with Paget’s without a palpable breast mass,
mammography has been reported as normal in 2.5% to 100% of patients. Of the 324 patients in the 11 series, 174 (54%) had normal mammograms (2, 13, 15, 16, 23). In nine series, breast histology was evaluated for 144 patients with clinical Paget’s disease and normal mammograms, with 29 patients (20%) found to have an associated invasive breast cancer, 111 patients (77%) found to have DCIS, and 4 patients (3%) found to have Paget’s disease of the nipple without an associated invasive cancer or DCIS (15, 23). These retrospective studies included patients accrued in the late 1970s, when xeromammography was still in use and retroareolar spot compression views were not routine.
mammography has been reported as normal in 2.5% to 100% of patients. Of the 324 patients in the 11 series, 174 (54%) had normal mammograms (2, 13, 15, 16, 23). In nine series, breast histology was evaluated for 144 patients with clinical Paget’s disease and normal mammograms, with 29 patients (20%) found to have an associated invasive breast cancer, 111 patients (77%) found to have DCIS, and 4 patients (3%) found to have Paget’s disease of the nipple without an associated invasive cancer or DCIS (15, 23). These retrospective studies included patients accrued in the late 1970s, when xeromammography was still in use and retroareolar spot compression views were not routine.
Mammographic findings include skin, nipple, and areolar thickening, nipple retraction, subareolar or more diffuse malignant microcalcifications, and a discrete mass or architectural distortion (24). Mammography inadequately determines the existence of underlying disease in patients with Paget’s. In addition, it cannot map the true distribution of the underlying pathology and, therefore, has limited value in determining the appropriate surgical procedure (24). In a more recent series from Memorial Sloan Kettering in 23 patients who had a negative mammogram, 65% had an underlying malignancy confined to the central quadrant of the breast implying that perhaps a negative mammogram may indicate suitability for breast conservation. Although the data is encouraging, the overall sensitivity of mammograms in this series was only 34%. However, if the mammogram was positive, it accurately predicted the extent of disease in 82% of patients, supporting the role of mammography in treatment planning (25).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree