Keywords
endocrine system, follicle, follicle activation, follicle death, folliculogenesis, ovarian hormones, ovarian reserve, ovary
1.1
Overview of Ovarian Function
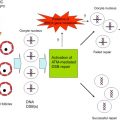
The ovary serves two roles – the production of hormones necessary to support the endocrine health of the individual and the generation of mature oocytes that are able to be fertilized and contribute half of the genetic makeup of a new organism. The ovarian follicle is the functional unit of the ovary that carries out both of these goals. The ovarian follicles within an adult ovary produce steroid and protein hormones in cyclical patterns in response to tropic factors from the hypothalamus and pituitary ( Figure 1.1 ). These hormones then act locally to support follicle and oocyte growth and development, as well as systemically to support reproductive health, bone health and cardiovascular function. Interruption of the endocrine hormones at the level of the brain or gonad due to cancer treatment can result in loss of cyclicity and diminished endocrine health for short periods of time or can render an individual sterile due to loss of ovarian follicles.
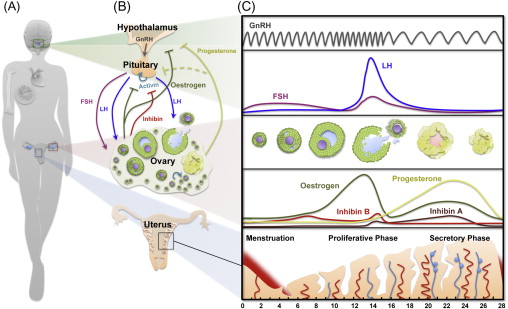
Each ovarian follicle consists of one oocyte surrounded by supporting somatic cells, the granulosa and theca cells, and the follicle structure is embedded in an ovarian stroma that provides additional critical signals that determine follicle fate. Follicle development starts with selection of follicles from a pool of dormant primordial follicles – the ovarian reserve. The recruited follicles are activated and undergo a series of changes in morphology and size, developing through primary and secondary stages before acquiring a fluid-filled antral cavity. Although multiple follicles are selected to begin growing and eventually reach the antral stage, many are lost to atresia and only one (or a few depending on the species) reaches the Graffian follicle stage. Ovulation occurs in response to a timed surge of pituitary gonadotropins (see Figure 1.1 ) that triggers the release of a mature oocyte from the surface of the ovary into the fallopian tube, where it can be fertilized. The residual follicle unit (the remaining theca and mural granulosa cells) undergoes transformation to become the corpus luteum.
Ovarian follicle development is a carefully orchestrated event that is directed by both endocrine signalling within the hypothalamic–pituitary–ovarian axis as well as paracrine signalling occurring within and between follicles in the ovary. Hormone-regulated follicular development involves the integration of feedback loops between oestrogen, progesterone, inhibin A and inhibin B from the ovary; follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the pituitary; and hypothalamic gonadotropin-releasing hormone (GnRH) to direct follicle and oocyte development (see Figure 1.1 ). On the other hand, early follicle development is driven by intrinsic ovarian factors that regulate oocytic and follicular development before the follicles acquire FSH responsiveness. These locally acting factors and their roles in maintaining the dormant follicle pool, activating selected primordial follicles and supporting the earliest stages of follicle development are less well understood and of intense interest in the reproductive field. Understanding the early signalling events associated with maintaining a health-quiescent follicle pool may provide insights into gonadotoxicity and how to mitigate this effect.
1.2
Ovarian Development
The mammalian gonad is formed during embryonic development ( Figure 1.2 ) after migration of primordial germ cells (PGCs) from the hindgut into the genital ridge between 9 to 10.5 days post coitum (dpc) in the mouse and at 6 weeks of gestation in humans. During this process, PGCs undergo mitotic divisions with incomplete cytokinesis resulting in cysts where germ cells remain connected by cytoplasmic bridges. At this stage, the gonad is bipotent. In the presence of a Y chromosome, the expression of the Sry gene (sex-determining region on the Y chromosome) directs male development through a process called sex determination. In contrast, sex determination in females requires the activity of several genes, such as Wnt4 , Rspo1 , β-Catenin , Foxl2 and Fst . Loss of these genes during development causes various degrees of female-to-male sex reversal.
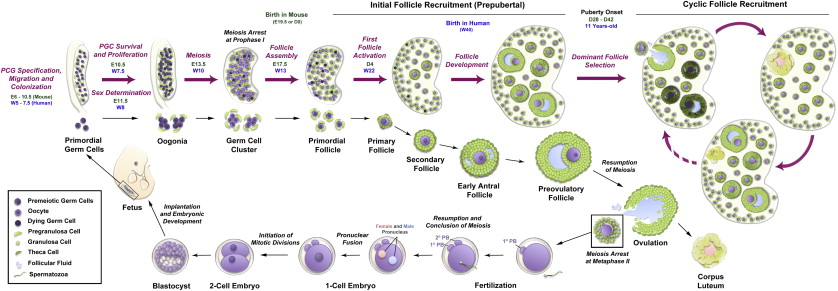
Following female-specific gene activation, the early ovary develops distinct structural features, including the formation of ovigerous cords by interaction of the germ cell clusters with surrounding pregranulosa cells. Around 13.5 dpc, mouse germ cells enter meiosis in an anterior-to-posterior gradient believed to be driven by retinoic acid (RA) ; in the human ovary, this process begins in the medulla and spreads radially into the cortex from 10 weeks gestation. Reductive division (meiosis) begins during embryonic development in the female gonad, and postnatally in the male germ cell, respectively. Although the developing testis is also exposed to RA, the production of CYP26B1 allows degradation of RA and the male germ cells remain arrested in G1/G0. Oogonia enter the first stages of meiosis and arrest in diplotene of prophase I; at this time, they are referred to as oocytes and remain arrested in this stage until the LH surge that triggers ovulation, with the final stages of meiotic division completed upon fertilization.
The newly formed oocytes become individually encapsulated in a single layer of somatic cells to form primordial follicles (see Figure 1.2 ). In contrast to humans and other large mammals, most primordial follicles in rodents are formed a few days after birth, with some being activated soon after formation. This process of primordial follicle formation is also called nest breakdown because the pregranulosa cells invade and degrade the cytoplasmic bridges within the cysts while some germ cells are systematically eliminated. Several factors have been identified that control the timing and extent of nest breakdown, including Figla , Foxl2 , Nobox , c-Kit (oocyte)/ Kit ligand (pregranulosa and granulosa cells), members of the transforming growth factor beta (TGFβ) superfamily, oestrogens and phytoestrogens, progesterone, extracellular matrix factors, neurotrophins, and members of the Notch signalling pathway.
Any insults that occur during ovarian embryonic development may negatively impact adult ovarian function; these include poor nutrition, exposure to environmental pollutants, and exposure to certain drugs. Thus, ovarian development represents a critical window of vulnerability that can influence normal gonadal function in the adult animal.
1.2
Ovarian Development
The mammalian gonad is formed during embryonic development ( Figure 1.2 ) after migration of primordial germ cells (PGCs) from the hindgut into the genital ridge between 9 to 10.5 days post coitum (dpc) in the mouse and at 6 weeks of gestation in humans. During this process, PGCs undergo mitotic divisions with incomplete cytokinesis resulting in cysts where germ cells remain connected by cytoplasmic bridges. At this stage, the gonad is bipotent. In the presence of a Y chromosome, the expression of the Sry gene (sex-determining region on the Y chromosome) directs male development through a process called sex determination. In contrast, sex determination in females requires the activity of several genes, such as Wnt4 , Rspo1 , β-Catenin , Foxl2 and Fst . Loss of these genes during development causes various degrees of female-to-male sex reversal.
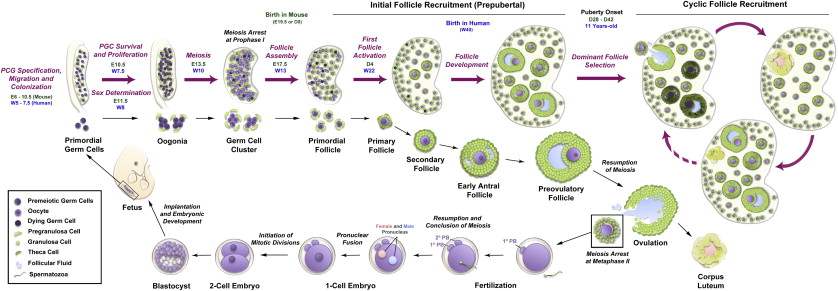
Following female-specific gene activation, the early ovary develops distinct structural features, including the formation of ovigerous cords by interaction of the germ cell clusters with surrounding pregranulosa cells. Around 13.5 dpc, mouse germ cells enter meiosis in an anterior-to-posterior gradient believed to be driven by retinoic acid (RA) ; in the human ovary, this process begins in the medulla and spreads radially into the cortex from 10 weeks gestation. Reductive division (meiosis) begins during embryonic development in the female gonad, and postnatally in the male germ cell, respectively. Although the developing testis is also exposed to RA, the production of CYP26B1 allows degradation of RA and the male germ cells remain arrested in G1/G0. Oogonia enter the first stages of meiosis and arrest in diplotene of prophase I; at this time, they are referred to as oocytes and remain arrested in this stage until the LH surge that triggers ovulation, with the final stages of meiotic division completed upon fertilization.
The newly formed oocytes become individually encapsulated in a single layer of somatic cells to form primordial follicles (see Figure 1.2 ). In contrast to humans and other large mammals, most primordial follicles in rodents are formed a few days after birth, with some being activated soon after formation. This process of primordial follicle formation is also called nest breakdown because the pregranulosa cells invade and degrade the cytoplasmic bridges within the cysts while some germ cells are systematically eliminated. Several factors have been identified that control the timing and extent of nest breakdown, including Figla , Foxl2 , Nobox , c-Kit (oocyte)/ Kit ligand (pregranulosa and granulosa cells), members of the transforming growth factor beta (TGFβ) superfamily, oestrogens and phytoestrogens, progesterone, extracellular matrix factors, neurotrophins, and members of the Notch signalling pathway.
Any insults that occur during ovarian embryonic development may negatively impact adult ovarian function; these include poor nutrition, exposure to environmental pollutants, and exposure to certain drugs. Thus, ovarian development represents a critical window of vulnerability that can influence normal gonadal function in the adult animal.
1.3
Molecular Mechanisms Controlling Primordial Follicle Activation
The primordial follicles are the first class of follicles formed in mammalian ovaries and consist of an oocyte surrounded by a single layer of flattened granulosa cells. These follicles remain in a dormant state until they receive signals for activation or are relieved of a negative regulatory factor. Dormancy is characterized by oocytic meiosis arrest at prophase I, pregranulosa G1/G0 mitotic arrest, and a relatively cortical location within the ovary with little or no vascular access. Each primordial follicle has four possible fates: remain quiescent, die by attrition, begin development but later be lost by atresia, or begin development and ultimately release an oocyte followed by formation of a short-lived corpus luteum (see Figure 1.2 ). A small number of primordial follicles are continuously activated to exit the ovarian reserve and begin growing. When development begins, the oocyte expands in size as metabolic activity increases and the surrounding pregranulosa cells start to proliferate and differentiate. Since follicle activation is irreversible, and the starting pool of follicles resulting from nest breakdown is finite, follicle activation is tightly controlled to permit continuous waves of follicle growth during the ~40 years of the female reproductive lifespan. It is believed that the trigger(s) for primordial follicle activation are intraovarian signals, but it is still unknown if signals originate in the oocyte or the granulosa cells. Many studies have identified important molecular players controlling global primordial follicle activation; however, the mechanisms underlying selective follicular activation over decades of life – namely, how certain primordial follicles enter the growth phase while others remain quiescent – are still largely unknown. One popular explanation for the order of follicle activation is the “production line” hypothesis. This hypothesis suggests that the first oogonia to undergo meiotic arrest are the first to become activated, implying that the order of follicle activation is established during embryonic development. However, this hypothesis remains controversial, with evidence both in favour and against.
Early studies showed that primordial follicles spontaneously activate when cultured in vitro , suggesting that quiescence is based on inhibitory factors present in vivo , although a stimulatory effect of factors in the culture medium could not be excluded. Indeed, a number of growth factors have been shown to be important for primordial follicle recruitment and maintenance, including vascular endothelial growth factor (VEGF), leukaemia inhibitor factor (LIF), basic fibroblast growth factor (bFGF), keratinocyte growth factor (KGF), platelet-derived growth factor (PDGF), neurotrophins, bone morphogenetic protein-4 (BMP-4), and BMP-7 and c-Kit/Kit ligand.
In vitro studies have shown that c-Kit/Kit ligand signalling activates the PI3K signalling network in oocytes, promoting their survival and growth. Multiple studies have demonstrated the importance of the PI3K–PTEN–AKT–mTOR signalling pathway in controlling primordial follicle dormancy. Transgenic animals in which Foxo3 , Tsc1 and Tsc2 , PTEN , and p27kip1 have been genetically ablated undergo premature ovarian failure (POF) due to global follicle activation. Genetic depletion of PDK1 (phosphoinositide-dependent kinase 1) and RpS6 (ribosomal protein 6) also causes POF, although it occurs through accelerated follicle clearance and atresia, suggesting that a certain level of PDK1 expression is important to control the balance between primordial follicle activation and dormancy. Manipulation of the PI3K pathway has been studied as an approach to induce activation of mouse and human primordial follicles in vitro ; however, a recent study noted detrimental effects of this strategy on follicle development and further safety studies will be required before application to humans. Taken together, these studies indicate that the PI3K signalling pathway is a critical determinant of primordial follicle activation and survival.
Other transcriptional factors also contribute to follicle activation. Forkhead Box L2 ( FoxL2 ) expression in granulosa cells has been implicated in primordial follicle activation by inducing the transition from squamous to cuboidal granulosa cells. Despite delayed nest breakdown, FoxL2 knockout mice form individual primordial follicles, which then arrest and undergo atresia at early stages of development, resulting in premature ovarian failure by 2 months of age. Blockage at the primordial follicle stage and accelerated germ cell loss have also been observed after genetic deletion of Nobox , Lhx8 , Sohlh-1 (spermatogenesis and oogenesis helix-loop-helix 1) and Sohlh-2 .
Others have proposed that substances produced by growing follicles, including anti-Müllerian hormone (AMH; also called Müllerian inhibitory substance [MIS]), exert an inhibitory influence on primordial oocytes, keeping them in a dormant state. AMH is also a useful biomarker of growing follicles and can be used for assessment of the ovarian reserve in patients following treatment for cancer ; this is reviewed in Chapter 3 . AMH produced by the granulosa cells of growing follicles appears to inhibit primordial follicle recruitment, as mice lacking AMH have an elevated number of growing follicles and show depletion of the primordial follicle pool earlier than their wild-type littermates. Other factors, such as the neuropeptide somatostatin (SST) and the chemoattractive cytokine CXCL12, are also known to inhibit the recruitment of primordial follicles.
In addition to the lack of understanding of the mechanisms controlling selective follicle recruitment, the extent and timing of the first wave of follicle activation (first growing follicles in the prepubertal ovary) are still largely unknown. Interestingly, recent work suggests that the timing of granulosa cell specification may contribute to fundamental differences between the initial and subsequent cyclical waves of primordial follicle recruitment and the maintenance of primordial oocyte quiescence. The first wave of growing follicles reaches the antral stage before puberty; in the absence of FSH, these follicles are believed to be unable to develop further and therefore lost to atresia. However, a recent study in mice challenged this concept by showing that these first-wave follicles not only induce the onset of puberty but also grow faster than follicles in the adult ovary and are ovulated. Further studies focusing on the similarities and differences between first and consecutive waves of primordial follicle activation are important to understand the molecular mechanisms controlling selective follicle recruitment.
1.4
Local Control of Early Follicle Development
After a primordial follicle has been activated and growth is initiated, the fate of the follicle is controlled by various interacting endocrine, paracrine, and autocrine factors. Preantral follicle growth is gonadotropin independent, as revealed in mutant mice with compromised FSH signalling that showed follicle development arrested only prior to antrum formation. The rate of early follicular development is dependent on the oocyte, which produces several growth factors, such as GDF9 and BMP15, that act on the granulosa cells. Follicles from mice null for these growth factors fail to progress to later stages of development. Conversely, overexpression of BMP15 causes an early decline in the ovarian reserve by accelerating follicle growth, suggesting that BMP15 is important for controlling follicle growth and preventing maturation. Bidirectional communication between the oocyte and its surrounding somatic cells plays a critical role in follicular development. Gap junctions between the oocyte and the supporting granulosa cells facilitate an exchange of signalling molecules during folliculogenesis. Deletion of connexin 37 (oocyte–granulosa cell gap junctions) and connexin 43 (granulosa–granulosa cell gap junctions) causes defects in oocyte meiotic competence and follicle growth arrest at the primary stage, respectively. Other factors have been shown to be important for early follicle growth, such as cyclin D2, IGF-1, and TAF4B. Additionally, formation of the theca cell layer is a crucial feature of early follicle growth. This cell type is believed to be differentiated from mesenchymal precursors present in the ovarian stroma and is the primary source for androgen production.
1.5
Endocrine Control of Later Follicle Development
During the preantral to antral transition, scattered fluid-filled spaces between granulosa cells converge to form a single antral cavity (see Figures 1.1 and 1.2 ). Accumulation of follicular fluid causes a rapid expansion in follicle size, while the theca layer differentiates into theca interna (steroidogenic activity) and theca externa (highly vascularized containing macrophages, smooth muscle-like cells and fibroblasts) compartments. The formation of the antrum also marks the differentiation of the granulosa cell layer into mural granulosa cells and cumulus cells. Antral follicles become receptive to extraovarian regulation and endocrine signalling within the hypothalamic–pituitary–gonadal (HPG) axis. The hypothalamus produces and releases pulses of GnRH into the pituitary blood supply, inducing pituitary release of FSH and LH in response to low- or high-frequency pulses, respectively (see Figure 1.1 ). FSH is essential for granulosa cell proliferation, oestradiol production, LH receptor expression and the prevention of granulosa cell apoptosis (atresia). Follicular oestrogens and inhibins exert negative feedback on the HPG axis that ultimately triggers an LH surge. During each wave of folliculogenesis, most of the recruited follicles are lost to atresia at the antral stage, while only a few (or one in humans) grow further to reach the preovulatory stage. When the follicle reaches the final stages of maturation, the LH surge induces meiotic resumption in the oocyte, breakdown of the follicle wall and extrusion of the cumulus–oocyte complex by ovulation. The remaining granulosa cells in the postovulatory follicle undergo luteinization and form a corpus luteum. This transient endocrine organ produces progesterone, which is essential for maintenance of pregnancy. Any insult affecting the activity of the HPG axis can compromise the oestrus/menstrual cycle and cause severe disruption of folliculogenesis and infertility.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree