Although epithelial ovarian cancer typically spreads in a locoregional fashion to involve the peritoneal cavity and retroperitoneal nodes, it can be found outside the abdomen as well. The most common site of extra-abdominal spread is the pleural space (thought to occur via transdiaphragmatic lymphatics), where it causes a malignant pleural effusion in some patients. Hematogenous metastases to the liver, spleen, or lung can also occur during the course of the disease, but are relatively uncommon at presentation. Bone or central nervous system metastases may rarely be observed in patients who have lived for many years after initial diagnosis, during which unusual patterns of disease spread may occur.
Histologic Classification of Epithelial Tumors
Table 76.1 outlines the classification of common epithelial tumors that has been accepted by the World Health Organization (WHO) and the International Federation of Gynecology and Obstetrics (FIGO).4 The nomenclature for these tumors reflects the cell type, location of the tumor, and degree of malignancy, ranging from benign lesions to tumors of low malignant potential (LMP) to invasive carcinomas.
Tumors of LMP (borderline tumors) have an excellent prognosis compared with invasive carcinomas.12,13 They are characterized by epithelial papillae with atypical cell clusters, cellular stratification, nuclear atypia, and increased mitotic activity. In contrast to epithelial ovarian carcinoma, borderline tumors lack stromal invasion.13
Epithelial carcinomas are characterized by histologic cell type and degree of differentiation (tumor grade). The histologic cell type has limited prognostic significance independent of clinical stage, although patients with clear cell and mucinous types of epithelial ovarian cancer fare less well due to the relative chemoresistance of these histologies.14 Conversely, low-grade serous carcinoma is associated with a better prognosis despite relative chemoresistance.15,16 High tumor grade appears to be an important prognostic factor, especially in patients with early stage epithelial tumors.
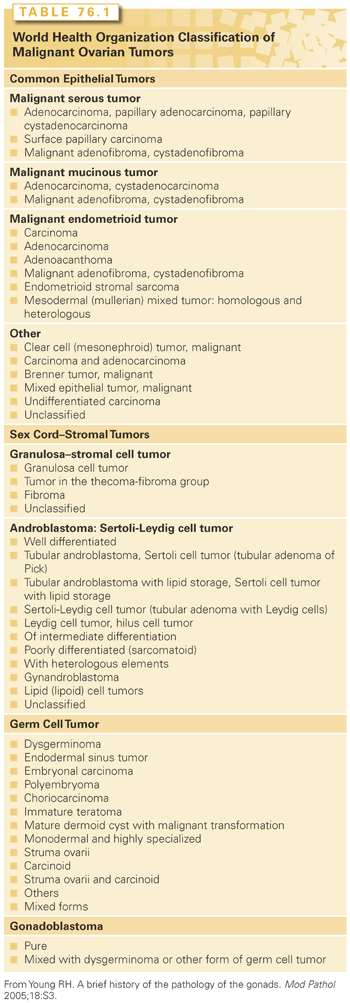
Certain pathologic and clinical features are characteristic of distinct histologic subtypes of epithelial carcinoma (Table 76.2). For instance, concentric rings of calcification called psammoma bodies (Fig. 76.2) are often observed in the papillary serous variety of epithelial ovarian cancer, although they are not pathognomonic for this disease and may also be seen, for example, in breast, lung, and papillary thyroid cancers. The endometrioid variant of ovarian cancer is associated with endometriosis in about 20% to 30% of cases, with a separate endometrioid uterine cancer (often stage I and low grade) simultaneously present in 15% of cases.17 Likewise, clear cell histology may also be associated with endometriosis, as well as humorally mediated hypercalcemia (which can also be observed with the rare small cell variant of ovarian cancer). Clear cell cancers are relatively resistant to chemotherapy compared to their more common papillary serous counterparts. Finally, primary mucinous ovarian cancers are also relatively chemoresistant, are sometimes associated with pseudomyxoma peritonei, and may not be associated with dramatic elevations of the cancer antigen (CA) 125 serum tumor marker.

Gastric, breast (especially infiltrating lobular carcinoma), mesothelioma, and colorectal cancers may occasionally present with diffuse peritoneal implants, ascites, and ovarian metastases that mimic primary ovarian cancer. It is usually possible to distinguish between these possibilities on routine light microscopic histologic evaluation, although immunohistochemistry can be most helpful when the histologic diagnosis is ambiguous. Staining for cytokeratin CK7 is positive and CK20 is negative in most cases of primary serous ovarian cancer, whereas the reverse staining pattern is typically observed for colorectal cancer. Staining for gross cystic disease fluid protein (GCDFP) may be positive in up to 50% of patients with breast cancer, whereas this marker should be negative in patients with gastric, colorectal, or ovarian cancer. Finally, calretinin is usually expressed in mesothelioma but is typically negative in epithelial ovarian cancer.
Diagnosis
Most patients with epithelial ovarian cancer experience no signs or symptoms of the disease until it spreads to the upper abdomen. Approximately 70% of patients with this tumor present with advanced disease (stage III or IV, Table 76.3), whereas the majority of patients with borderline, germ cell, and sex cord–stromal tumors present with early stage disease limited to the pelvis.2 Occasionally, patients with epithelial ovarian cancer will be diagnosed with early stage disease due to discovery of a mass on routine pelvic examination or because of pelvic pain caused by ovarian torsion. Unlike epithelial cancers, which are generally asymptomatic at an early stage, ovarian germ cell malignancies tend to stretch and twist the infundibulopelvic ligament, causing severe pain while the disease is still confined to the ovary.
Abdominal discomfort, bloating, and early satiety are the most common symptoms experienced by women with epithelial ovarian cancer. Patients presenting with such nonspecific complaints may be found to have ascites and a pelvic mass on physical examination. Occasionally an umbilical lymph node metastasis will be present (Sister Mary Joseph node) or a pleural effusion will be found. The mass on pelvic examination is frequently firm and fixed, with multiple nodularities palpable in the cul-de-sac.
The CA 125 serum level is elevated in >80% of serous epithelial ovarian cancers.18 However, it is not a reliable diagnostic test, as it can also be elevated in a variety of benign gynecologic conditions (such as endometriosis, pelvic inflammatory disease, or pregnancy) and nongynecologic malignancies (such as breast, lung, and gastrointestinal cancers). Furthermore, the CA 125 level is elevated in only approximately 50% of patients with early stage epithelial ovarian cancer, which also limits its value as a screening test.18 Other tumor markers, such as CA 19-9, which is elevated in some mucinous ovarian carcinomas, and carcinoembryonic antigen (CEA) are less frequently useful. It is typical for a patient with epithelial ovarian cancer to have a normal CEA level in the setting of a significantly elevated CA 125 level. Postoperatively, the CA 125 level provides a sensitive way to monitor treatment response and development of disease recurrence. Because relapsed epithelial ovarian cancer is usually incurable, however, there is currently no evidence that early detection of recurrence through CA 125 surveillance confers either a quality of life or a survival advantage in this disease.19
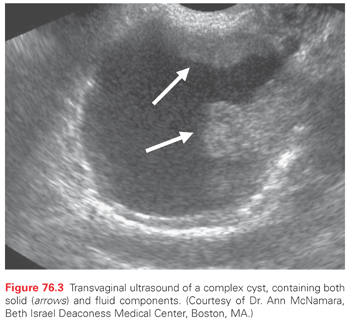
Transvaginal ultrasonography (TVU) is an important diagnostic tool in the evaluation of patients with a pelvic mass. TVU is more sensitive at detecting ovarian tumors compared to other tests such as computed tomography (CT), and it can provide qualitative information about the mass that might suggest malignancy. The classic sonographic finding of malignancy is a “complex” cyst, defined as containing both solid and cystic components, sometimes with septations and internal echogenicity (Fig. 76.3). Finding a complex cyst on sonography, especially in the presence of signs and symptoms consistent with ovarian cancer, often requires surgery for further evaluation. It is best to avoid percutaneous biopsy during the initial evaluation, which can result in cyst rupture and spillage of malignant cells into the peritoneal cavity. Bilateral ovarian involvement and ascites are sometimes detected by sonography as well. Color Doppler imaging evaluates blood flow to an ovarian mass and can potentially identify a malignant process based on the presence of abnormal neovascularization.20
In contrast to complex cysts, “simple” cysts are defined as being thin-walled, fluid-filled, without a mass component, septations, or internal echogenicity on TVU examination. Simple cysts are most often benign in nature and may be found in 5% to 10% of asymptomatic postmenopausal women during TVU examination, especially in the first decade after menopause. Simple cysts do not always require surgical evaluation if they are associated with normal CA 125 levels, although management must be individualized.21 Postmenopausal women with simple cysts in association with elevated serum CA 125 levels, simple cysts that are >5 to 10 cm in diameter, or simple cysts in association with abnormal color Doppler flow studies are often referred for surgery.
In premenopausal women, simple cysts detected on TVU examination may be functional (i.e., a corpus luteum cyst) or represent a benign process such as a serous cystadenoma. Such cysts may generally be followed through several menstrual cycles, during which they often resolve. Functional cysts may also disappear when oral contraceptives are used. However, premenopausal women with simple cysts that are persistent or enlarging, especially in the setting of a rising CA 125 level, are reasonable candidates for surgical evaluation to exclude malignancy.21 As previously mentioned, several benign conditions in premenopausal women may also be associated with elevated CA 125 levels, such as pregnancy or endometriosis, and there is no absolute CA 125 cutoff to distinguish benign from malignant pathologies.22
CT or magnetic resonance imaging may sometimes be helpful in defining the extent of peritoneal disease in patients with suspected ovarian cancer. However, for the patient with a complex ovarian cyst and clinical signs and symptoms to suggest ovarian cancer, these studies generally do not obviate the need for surgical exploration. Occasionally, CT may sometimes be helpful in distinguishing a gynecologic malignancy from a metastatic pancreatic neoplasm, for instance, for which an exploratory laparotomy may not be warranted. In selected patients, CT may also assist in surgical planning by locating the site of suspected bowel obstruction. Magnetic resonance imaging has not been shown to have a clear advantage over CT in patients with an ovarian mass, except for pregnant patients when ultrasonography is inconclusive and there is a desire to avoid radiation exposure. Positron emission tomography (PET) is a form of functional imaging that most frequently uses the positron-emitting glucose analogue fluorodeoxyglucose. Tumor masses are imaged based on their relatively increased glucose metabolism compared to normal tissues. However, there is currently no proven role for PET in the diagnosis or subsequent follow-up of patients with ovarian cancer.23 Chest radiographs may sometimes be performed to evaluate the presence of pleural effusions, which occur in 10% of patients with epithelial ovarian cancer at diagnosis.
Screening and Early Detection
A successful screening test for ovarian cancer should be capable of identifying the majority of patients with precancerous lesions or early disease. Because a positive screening test for ovarian cancer would result in major surgery with associated morbidity, costs, and even mortality, the false-positive rate of such a screening test must be low, and its positive predictive value (PPV) relatively high (at least 10%). At present, there are no screening tests for epithelial ovarian cancer that convincingly meet these criteria.
Ovarian palpation has not been established as a useful screening procedure, and most screening studies have used serum tumor marker levels, ultrasonography, or both. The CA 125 serum level is not a useful screening test when used alone, as elevations are not specific for ovarian cancer and may be observed in cirrhosis, peritonitis, pleuritis, pancreatitis, endometriosis, uterine leiomyomata, benign ovarian cysts, and pelvic inflammatory disease. In addition, CA 125 serum levels may be elevated in other malignancies such as breast, lung, colorectal, pancreatic, and gastric cancers. Finally, although the CA 125 level is elevated in the majority of patients with advanced epithelial ovarian cancer, it is abnormal in only half of patients with early stage disease.18 Therefore, by itself this test would fail to detect a sizable fraction of patients with curable disease. More recently, a number of candidate markers have been discovered that show promise for enhancing the accuracy of CA 125 levels, such as human epididymis 4, osteopontin, mesothelin, and osteoblast-stimulating factor-2. Algorithms that define the behavior of these markers have also been developed, incorporating biologic characteristics of tumor growth and marker behavior.24 Levels of OVX-1 and macrophage colony-stimulating factor have been found to be elevated in patients with clinically evident ovarian cancer but normal CA 125 levels, which suggests that these markers may be complementary to CA 125.25 Lysophosphatidic acid level has also been reported to discriminate patients with ovarian cancer from controls, including cases with early stage disease.26 None of these tests has been proven to have sufficient sensitivity and specificity for routine screening at the current time.
Measurement of the CA 125 level has been combined with performance of TVU in an attempt to improve screening.20,27,28 Early studies of TVU suggested a sensitivity of close to 100% but a specificity of 98%, which is insufficient to achieve a PPV of 10%. More recent reports suggest that use of color Doppler imaging improves the specificity of TVU, but it is uncertain whether this will achieve the desired PPV. Investigators at the University of Kentucky improved the specificity of TVU by using a morphologic index. They screened 6,470 women, including high-risk premenopausal women and average-risk postmenopausal women.28 Of 90 women who underwent surgery, 6 were found to have an ovarian malignancy, for a PPV of 6.7%. One interval cancer was found at prophylactic oophorectomy 11 months after screening, for a sensitivity of 86%. All but one of these cancers were stage I, and no deaths due to ovarian cancer were noted in this group.
Two randomized controlled trials are currently under way to evaluate a multimodal screening approach using both CA 125 and TVU. In the United States, the Prostate, Lung, Colorectal, Ovarian Cancer Screening Trial uses measurement of CA 125 level (single threshold elevation of >35 U/mL) and TVU together, performed annually, as a first-line screen.29 If either test is positive, the woman is referred for surgical consultation. In this two-arm, randomized controlled trial involving 10 centers, 78,216 of general US women aged 55 to 74 years were randomly assigned to the screening arm or to a standard-care control arm. After a median follow-up of 12.4 years, no mortality reduction was observed for patients randomized to the screening arm.30
The second randomized screening trial is currently being conducted in the United Kingdom and uses CA 125 levels (or rate of rise of CA 125) as a trigger for performing TVU. This trial is based on an earlier study by Jacobs et al.31 in which 21,935 average-risk postmenopausal women were assigned to undergo three annual screenings or no screening. The screening protocol used CA 125 level as a first-line screen and referred the patient for TVU if the CA 125 level was >30 U/mL. If the TVU revealed an ovarian mass, the patient was referred for surgical consultation. Findings from this trial support the notion that this stepwise approach can yield high specificity and an acceptable PPV.31 Specifically, when the decision rule for surgical referral requires that results of both tests be positive, the PPV is 20%. Furthermore, there were one-half as many deaths in the screened group as there were among controls, and there was a statistically significant improvement in survival. Individuals with index cancers survived an average of 72.9 months in the screening group and 41.8 months in the control group. However, the multimodal screening strategy originally described by Jacobs et al.31 is limited by the sensitivity of the CA 125 level, which serves as a trigger for performing ultrasound. Accordingly, these investigators are exploring ways to improve on these results by detecting changes in CA 125 levels over time. Skates et al.32 suggested fitting an exponential model that uses data from several prior CA 125 screens, with an exponential rise triggering a callback for additional ultrasound testing. This screening strategy forms the basis of the three-arm randomized trial currently being conducted in the United Kingdom.
Hereditary Ovarian Carcinoma
Approximately 5% to 10% of patients with epithelial ovarian carcinoma carry a germline mutation that places them at substantially increased risk of developing this disease. The breast–ovarian cancer syndrome accounts for approximately 90% of hereditary ovarian cancer and is often suspected whenever the pedigree reveals multiple affected family members with ovarian cancer, bilateral or early onset breast cancer, both breast and ovarian cancer in the same individual, or a male relative with breast cancer.33,34 Fallopian tube cancer and primary peritoneal serous cancer (PPSC) are also recognized to be part of this syndrome.35 The high incidence of breast and ovarian cancers in these families is due to inherited germline mutations in the BRCA1 or BRCA2 genes, which may be transferred by either parent, meaning that both maternal and paternal family histories must be obtained to determine risk.36 The BRCA1 gene, located on chromosome band 17q12-21, and the BRCA2 gene, located on chromosome band 13q12-13, were identified and linked to hereditary breast and ovarian cancers in the 1990s. Emerging evidence suggests that these genes act as tumor suppressors and play a critical role in the repair of double-stranded DNA breaks.36
Many mutations have been described throughout the BRCA1 and BRCA2 genes, with nonsense and frameshift mutations being predominant.36 Nonsense mutations occur when a nucleotide substitution results in a stop codon, and frameshift mutations occur when one or more nucleotides are deleted to produce a downstream stop codon. Certain ethnic groups have higher frequencies of distinctive BRCA mutations, thought to be due to a founder effect in which certain mutations are preserved within a genetically isolated population. Three such founder mutations (185delAG BRCA1, 5382insC BRCA1, and 6174delT BRCA2) are carried by 2% to 2.4% of the Ashkenazic Jewish population.37 Furthermore, up to 40% of patients of Jewish descent with epithelial ovarian cancer may carry one of these mutations (regardless of their family histories).37 This is compared to a carrier frequency of approximately 5% among non-Jewish women with ovarian cancer.
The lifetime risk of ovarian cancer is approximately 20% to 40% for patients with BRCA1 mutations, and 10% to 20% for BRCA2 mutation carriers.38 Ovarian cancer associated with germline mutations of BRCA1 appears to present with distinct clinical and pathologic features compared with sporadic ovarian cancer.39 The majority of BRCA1-associated cancers are serous adenocarcinomas, with an average age at diagnosis of 48 years, whereas the mean age for BRCA2-associated ovarian cancers is 60 years.40 Other histologies may also occur, including endometrioid and clear cell tumors, although mucinous ovarian cancer appears to be underrepresented in these genetic syndromes. Furthermore, BRCA-associated cancers may have a more favorable course than sporadic ovarian cancer. In a study by Rubin et al.,39 the median survival of 43 patients with advanced BRCA1-associated disease was 77 months, compared with 29 months for matched controls. Cass et al.41 noted a similar survival advantage for carriers in their matched cohort study and suggested that this was a result of having an improved response to platinum-based chemotherapy compared to women with sporadic disease. This increased chemosensitivity may be partly due to the inability of tumor cells to repair platinum-induced DNA damage in the setting of a BRCA1 or BRCA2 mutation.36 The more favorable survival of patients with BRCA1 or BRCA2 mutations when compared to their sporadic counterparts is not necessarily related to a higher rate of cure, but may also be related to a longer duration of responsiveness to chemotherapy agents used in the relapsed setting. The Gynecologic Oncology Group (GOG) is conducting a prospective study to better compare the clinical course of sporadic ovarian cancer with that associated with BRCA1 and BRCA2 mutations.
The hereditary nonpolyposis colorectal cancer (HNPCC) syndrome accounts for approximately 5% to 10% of all hereditary ovarian cancer cases.17 It is an autosomal dominant genetic syndrome characterized by nonpolyposis colon cancer, often involving the right colon, as well as an increased risk of developing endometrial, ovarian, hepatobiliary, upper gastrointestinal, and genitourinary cancers.42 Colorectal and uterine cancers comprise the majority of tumors developing in affected families. The risk of endometrial cancer among women in HNPCC syndrome families is estimated to be 40% to 60% by age 70, compared to 1.5% in the general population. Limited studies have reported a 3.5-fold increase in the risk of ovarian cancer in members of these families.42 A germline mutation in one of five genes involved in DNA mismatch repair is responsible for the HNPCC phenotype: hMSH2 (chromosome arm 2p), hMLH1 (chromosome arm 3p), hPMS1 (chromosome arm 2q), hPMS2 (chromosome arm 7p), and hMSH6 (chromosome arm 2p).36 The majority of affected patients are found to have defects in either hMSH2 or hMLH1. Patients with HNPCC due to germline mutation of hMSH6 may be particularly predisposed to uterine cancer. In addition, HNPCC may account for approximately 7% of cases with synchronous uterine and ovarian cancers, which are often (but not always) low grade and of endometrioid histology.17 The estimated cumulative risks for ovarian cancer by age 70 years can be as high as 24% for those with germline mutations in MLH1 and MSH2.43
Patients at high risk of having a hereditary cancer typically undergo genetic counseling, so that the ramifications of genetic testing can be discussed. Multidisciplinary services available in such a setting often include pretest and posttest counseling, screening, treatment, and psychosocial counseling.44 The most direct approach to determine whether a cancer-associated mutation is present is to test the patient affected with the disease (the proband), because he or she is the most likely to carry a deleterious mutation. The first family member to be tested will often require comprehensive gene sequencing. Other individuals can then be tested for the identified mutation, which may be unique to this particular family. In the Ashkenazic Jewish population, genetic testing for the three founder mutations is required because the carrier frequency in this population is high, and individuals may occasionally harbor germline mutations in both BRCA1 and BRCA2.
Test results may reveal an identifiable mutation, no identifiable mutation, or a polymorphism of indeterminate clinical significance. If the proband has tested positive for a recognized mutation, then a relative with a negative result has likely not inherited the deleterious mutation, and her cancer risk approximates that of the general population. However, if no identifiable mutation is found in the proband, it is still possible that a cancer-associated mutation exists that is not detectable with current testing methods. This is especially the case for probands with a highly suggestive family history of breast, ovarian, or both cancers. In this regard, it has been shown that approximately 12% of probands who test negative for a germline mutation using standard gene sequencing techniques are found to have a clinically significant mutation in BRCA1 or BRCA2 when tested by the technique of multiplex ligation dependent probe amplification.45 Finally, a minority of test results represent genetic variants or polymorphisms in BRCA1 or BRCA2 that are of indeterminate clinical significance. Further study of these genetic variants and associated cancer risks in large populations will help reduce the number of reports of indeterminate findings.
The management of patients with an inherited genetic predisposition to ovarian cancer is complex due to the variable penetrance of genetic alterations and the lack of effective early detection methods for ovarian cancer. Although annual pelvic examinations, serum CA 125 determinations, and TVU are sometimes considered in affected individuals, there is currently no conclusive evidence that ovarian cancer mortality is reduced as a result of these interventions.27 In contrast, the efficacy of prophylactic, risk reduction bilateral salpingo-oophorectomy (RRSO) for patients with the hereditary breast–ovarian cancer syndrome has been more convincingly demonstrated.46,47 One study of 259 patients who underwent RRSO found a 96% decrease in ovarian cancers and an approximately 50% reduction in subsequent breast cancer compared to age-matched controls.46 More recent studies also confirm the benefit of RRSO in this patient population.48 Even after RRSO, BRCA mutation carriers are still at small risk for developing PPSC, which is histologically and clinically similar to epithelial ovarian cancer. Such cancers represent malignant transformation of the peritoneal mesothelium, which is contiguous with ovarian surface epithelium.
RRSO is generally considered for patients at high risk for developing ovarian cancer and who have completed childbearing, especially if they are at least 35 years of age.27,49 A laparoscopic approach is frequently possible, but the surgical options must be individualized, as must the decision for concomitant hysterectomy (which is an especially relevant consideration for patients with HNPCC undergoing this procedure). It is important to remove the fallopian tubes as part of prophylactic surgery, as the tubal epithelium may harbor dysplasia or may develop in situ cancers in this setting.50 The surgical pathologist must perform a careful examination of the surgical specimens, as occult ovarian and tubal carcinoma have been found in 2% to 10% of RRSO specimens.51 Some patients with occult disease discovered after careful pathologic evaluation may require a second operation to complete surgical staging in order to determine the need for postoperative treatment, as described later in the chapter. Significant issues regarding RRSO remain unresolved, such as the physiologic adjustments to premature surgical menopause and the safety of hormone replacement therapy in this group, especially in those at high risk for breast cancer.52
It has been suggested that chemoprophylaxis with oral contraceptives for 5 years might decrease ovarian cancer risk by 50% in both the general population and in high-risk women. For example, a case-controlled study of 207 known BRCA mutation carriers and their sister controls found a 60% reduction of ovarian cancer risk with oral contraceptive use.53 Other risk-reducing strategies such as tubal ligation and hysterectomy have also been associated with a reduced incidence of ovarian cancer among high-risk women.3 Nonetheless, RRSO is currently the most effective preventative strategy to reduce ovarian cancer risk in patients with BRCA mutations.
Staging
Exploratory laparotomy serves three main purposes in the management of patients with suspected ovarian cancer. First, laparotomy permits histologic confirmation of disease, as a complex cyst may not only represent primary ovarian cancer, but may also be caused by metastatic gastric cancer to the ovary (Krukenberg tumor), metastatic disease to the ovary from a gastrointestinal or breast primary (especially infiltrating lobular breast cancer), or benign conditions such as endometriosis.54 Surgery is also necessary to determine the extent of disease (staging), which is critical in determining whether postoperative treatment will be necessary, as well as to assess prognosis. Finally, exploratory laparotomy is necessary to permit debulking of as much tumor as possible, as patients who are optimally cytoreduced (defined as having ≤1-cm diameter residual tumor) have a better prognosis compared to those with greater amounts of residual disease.55
The staging system for ovarian cancer was updated in 2014 by FIGO and is based on the findings at exploratory laparotomy (see Table 76.3).56,56a Proper surgical staging via exploratory laparotomy requires a midline incision large enough to permit inspection of the peritoneal cavity, including the upper abdomen, as well as evaluation of the retroperitoneal spaces and lymph nodes.57 When the peritoneal cavity is entered, ascitic fluid is aspirated and the peritoneal surfaces are irrigated, with the samples sent for cytologic evaluation. The pelvis and paracolic spaces should be irrigated and the fluid sent for cytologic examination. If intraperitoneal (IP) carcinomatosis is absent, it may be most appropriate to first resect the ovarian tumor and then to proceed with surgical staging to avoid rupturing the mass. The grossly normal, opposite ovary may undergo biopsy, or any visible benign-appearing cysts may be excised. Pelvic and para-aortic retroperitoneal lymph nodes are generally evaluated in patients whose tumors do not grossly extend outside the ovary, as approximately 10% to 15% of patients with otherwise stage I disease will have occult nodal involvement that places them in the stage III category.57 Any enlarged pelvic retroperitoneal lymph nodes should be removed if technically feasible in order to achieve optimal cytoreduction.
It is frequently necessary to extend the vertical incision above the umbilicus in order to fully inspect the upper abdomen. If gross disease is not present in the omentum, an infracolic omentectomy is usually sufficient for diagnostic purposes. When the omentum demonstrates diffuse infiltration by tumor (an omental cake), it should be excised from the greater curvature of the stomach as completely as possible. The upper abdominal evaluation continues with a careful inspection of the right hemidiaphragm, liver serosa, and liver parenchyma. The spleen is then carefully inspected, as is the left diaphragm. A splenectomy could be considered if this procedure would lead to an optimal surgical cytoreduction. The paracolic spaces and large bowel are then carefully inspected. The small intestine and mesentery are evaluated, and any tumor implants are removed as much as possible. If luminal narrowing is present, especially in the area of the terminal ileum, a small bowel resection and reanastomosis are performed. Similarly, if tumor appears to invade the large bowel, a resection may be required if the mass is large enough to pose a threat for bowel obstruction. Lymphadenectomy is considered if this procedure is technically feasible and would lead to a maximally cytoreductive result. In postmenopausal women or in women in whom fertility is no longer desired, a bilateral salpingo-oophorectomy and total abdominal hysterectomy are typically performed.
For women who wish to preserve fertility, which is sometimes possible when the tumor is limited to one ovary, staging may be performed without removal of the contralateral ovary and tube and without hysterectomy.58 In that regard, patients with endometrioid ovarian cancer may have a synchronous endometrioid uterine cancer in up to 15% of cases, which may not always be appreciated preoperatively.17 In such cases, the tumor is often minimally invasive and low grade, and the prognosis is often favorable. Therefore, for patients with early stage, endometrioid ovarian cancer in whom a fertility sparing operation is considered, it is reasonable to perform an endometrial biopsy to exclude the presence of a separate uterine cancer that would alter the surgical approach.
On occasion, the initial surgical staging is incomplete due to lack of lymph node or upper abdominal evaluation in a patient with presumptive stage I disease. In this situation, it is reasonable to consider completing the surgical staging if the findings would alter postoperative management. For instance, if a patient has stage IA, grade 1 or 2 disease (see Table 76.3) after complete surgical staging, no further postoperative treatment would generally be indicated.59 However, if a patient is already known to have at least stage IC or stage II disease, or if the tumor is grade 3, then postoperative chemotherapy is generally required, and the impact of additional staging procedures might be less important (except perhaps for the patient who is discovered to have stage III disease on re-exploration, in which case IP chemotherapy might play a role if she is optimally cytoreduced (as noted subsequently)). Laparoscopic or robotic surgical techniques may allow para-aortic lymph node dissections and omentectomies to be performed with less morbidity, which is an important consideration in a patient who might have undergone recent, albeit incomplete, surgical evaluation. If this is not possible or practical, an alternative is to obtain a CT scan in an attempt to identify the presence of any subclinical bulky disease that might be amenable to surgical resection. However, CT is not capable of detecting small volume or microscopic disease that could affect decisions regarding the need for postoperative chemotherapy.
Prognostic Factors for Epithelial Ovarian Cancer
Clinicopathologic findings of prognostic value include FIGO stage, volume of residual disease after cytoreductive surgery, histologic subtype, histologic grade, age, and malignant ascites.14 Tumor stage remains the most important prognostic factor, although only 30% of patients have early stage disease (defined as having stage I or stage II tumors). Stage IA disease is completely encapsulated, without involvement of the ovarian surface, without rupture, malignant ascites, or positive washings (see Table 76.3). The 5-year survival of patients with stage IA disease and grade 1 or 2 histology is >90% after surgery alone, and several investigators include patients with stage IB, grade 1 or 2 disease, in this good prognostic group.59 However, the relapse rate without postoperative adjuvant treatment is 30% to 40% for patients with stage IC disease (defined by the presence of rupture, surface involvement, malignant ascites, or positive washings), those with stage I, grade 3 disease, or those with stage II tumors. These patients comprise a high-risk group of early stage tumors and experience a 5-year survival rate of approximately 80% after receiving postoperative adjuvant therapy.59 It should be noted that the prognostic significance of rupture as the sole criterion for stage IC disease is somewhat controversial, as some investigators report that rupture alone does not appear to confer a worse prognosis if it occurred intraoperatively, as opposed to preoperatively.60
The majority of patients with epithelial ovarian cancer present with advanced disease (stage III or IV). After postoperative treatment, the 5-year survival rate of patients with stage III optimally debulked, gross residual disease (≤1-cm diameter residual tumor) is approximately 20% to 30%, and this decreases to <10% for patients with suboptimally debulked stage III disease or those with stage IV tumors.61,62 Patients who have stage IIIA disease on the basis of microscopic upper abdominal involvement (usually detected on omental biopsy) have survivals in the range of 50% after postoperative adjuvant therapy.63 Patients with advanced-stage disease who have mucinous or clear cell histologic also have a worse prognosis, which appears to be related to the relative chemoresistance of these histologies. In a GOG study, no patient with advanced clear cell or mucinous histology achieved a pathologic complete response after chemotherapy, as defined by performance of second-look laparotomy.14
Preoperative serum CA 125 levels frequently reflect the volume of disease and do not appear to have an independent effect on survival, after correcting for stage and debulking status. However, postoperative CA 125 levels, both during and after completion of first-line chemotherapy, have prognostic value.64,65 Some investigators have demonstrated that normalization of the serum CA 125 levels after three cycles of chemotherapy is associated with more favorable outcome, as well as achievement of a CA 125 nadir of ≤10 U/mL upon completion of treatment.64,65 Although this information has prognostic significance, it has limited therapeutic value in the absence of effective salvage regimens with curative potential.
Germline mutation in BRCA1 or BRCA2 genes is associated with an improved prognosis. A pooled analysis of 26 observational studies on the survival of women with ovarian cancer showed that having a germline mutation in BRCA1 or BRCA2 was associated with improved 5-year overall survival (for BRCA1: hazard ratio [HR] = 0.73; for BRCA2: HR = 0.49).66 In addition, several other molecular prognostic factors have been investigated in ovarian cancer. These include markers of proliferation or drug resistance, levels of serum cytokines or growth factor receptors, and expression of genes associated with metastases.67 More recently, it has been possible to use microarray analysis, which provides a global snapshot of gene expression, to assess prognosis and response to therapy in this disease. At least two profiles have been defined, referred to as the Ovarian Cancer Prognostic Profile and the Chemo Response Profile, that provide information of independent prognostic and predictive value for patients with advanced disease.68,69 However, it remains to be determined how best to incorporate such new techniques into the management of patients with this tumor. In the future, it is hoped that gene expression profiling will be capable of identifying patients who might benefit from novel forms of treatment (such as antiangiogenic therapy) or those with poor prognosis who might be appropriate for clinical trial participation.
Management of Early Stage Disease
Postoperative Chemotherapy
The results of two randomized European trials (International Collaborative Ovarian Neoplasm Trial [ICON] 1 and Adjuvant ChemoTherapy in Ovarian Neoplasm Trial) suggest that adjuvant chemotherapy can improve both progression-free survival (PFS) and overall survival in patients with high-risk, early stage ovarian cancer.70 Such patients include those with stage I, grade 3, stage IC, or any stage II disease. These two studies collectively enrolled 925 patients, although they differed in eligibility criteria, requirements for surgical staging, and the specific adjuvant chemotherapy program used. Overall survival was superior for platinum-based adjuvant therapy in the entire patient cohort, although subset analysis revealed that the benefit appeared to be restricted to those patients who did not undergo adequate surgical staging. This observation suggested that the observed survival benefit might be due to unintentional enrollment of a subset of patients who actually had occult stage III disease. Nonetheless, caution must be used when interpreting the results of an unplanned subset analysis, and most physicians feel that these data support the use of adjuvant chemotherapy for patients with high-risk, early stage disease.71
A study performed by the GOG compared three cycles of carboplatin and paclitaxel to six cycles of this regimen in patients with high-risk, early stage ovarian cancer in order to determine whether a shorter duration of treatment is equally effective and less toxic.72 The relapse rate and PFS were not statistically different between these two treatment arms, which led some investigators to conclude that administering three cycles of adjuvant chemotherapy was as effective as six cycles in this setting. However, a recent subset analysis of this trial involving patients with serous histology showed that the 5-year recurrence-free survival for patients who received six cycles was 83%, compared with 60% for those who received three cycles (p = 0.007).73 In addition, this study was not sufficiently powered to determine whether three cycles was equivalent to six cycles in terms of an overall survival benefit. In view of these issues, administration of six cycles of adjuvant carboplatin plus paclitaxel chemotherapy can still be considered a reasonable approach for high-risk, early stage patients, unless more definitive data supporting lesser amounts of chemotherapy become available.74 The rationale for choosing carboplatin and paclitaxel is based on the experience gained from management of advanced disease, which will be described in detail later in the chapter.
Postoperative Radiation Therapy
The role of radiation therapy in the treatment of patients with primary ovarian cancer has changed markedly over time. For several decades, it was used as the sole modality of treatment. As experience with chemotherapy grew, randomized trials were conducted to compare different chemotherapy regimens to whole abdominal radiotherapy (WAR) or to IP administration of radioactive phosphorus (32P). Most are now of only historical interest, as the chemotherapy regimens used did not contain platinum compounds, and they will not be reviewed here. In general, these studies showed that WAR and 32P had similar efficacy compared to prolonged courses of melphalan or chlorambucil, given with or without pelvic irradiation.
The first trial to compare radiation therapy to a platinum-based regimen was conducted from 1981 to 1987 in Birmingham, England. This trial randomized 40 patients with stages IC to III ovarian cancer with no macroscopic residual disease following surgery to either WAR using a moving-strip technique or to cisplatin (100 mg/m2, given every 3 weeks for five cycles). With a median follow-up of 84 months, the 5-year survival rates in the two groups were 58% and 62%, respectively.75
A trial conducted in Genoa, Italy, from 1985 to 1989 included 70 patients with high-risk stage I or II ovarian carcinoma.76 They received either WAR using an open-field technique (24 fractions delivering 43.2 Gy to the pelvis and 30.2 Gy to the upper abdomen) or cisplatin (50 mg/m2) plus cyclophosphamide (600 mg/m2), given every 4 weeks for six cycles. Eight patients randomized to WAR received chemotherapy because of their own or their physicians’ preference. Because protocol compliance was poor and accrual low, the study was prematurely closed. At a median follow-up of 60 months, the 5-year overall survival rates in the radiation and chemotherapy arms were 53% and 71%, respectively (p = 0.16); relapse-free survival rates were 50% and 74%, respectively (p = 0.07). Of note, late bowel obstruction requiring surgery occurred in only one patient.
From 1982 to 1988, 340 eligible patients with stages I through III ovarian carcinoma without residual disease after surgery seen at the Norwegian Radium Hospital in Oslo were randomized to receive either IP radioactive chromic phosphate (7 to 10 mCi, or 260 to 370 MBq) or six cycles of intravenous (IV) cisplatin (50 mg/m2) given every 3 weeks.77 Twenty-eight patients with peritoneal adhesions who were randomized to IP 32P were treated instead with WAR. The 5-year overall survival rates in the two arms were 83% and 81%, respectively; disease-free survival rates were 81% and 75%, respectively. Patients with stage II or III tumors had superior survival when treated with cisplatin, although the difference was not statistically significant. The long-term risk of bowel obstruction without recurrence in radiation and chemotherapy arms was 11% and 2%, respectively.
Finally, a trial conducted between 1986 and 1994 by the GOG randomly allocated 229 eligible patients with stage IA-B grade 3 or stage II tumors without macroscopic residual disease after surgery to either IP 32P (15 mCi) or three cycles every 3 weeks of cyclophosphamide (1,000 mg/m2) and cisplatin (100 mg/m2).78 With a median follow-up of nearly 10 years in survivors, the 10-year relapse rates in the radiation and chemotherapy arms were 35% and 28%, respectively, which was not statistically significant. There was a relative reduction of 17% in the overall death rate in the chemotherapy arm, which was not statistically significant. The incidence of nonhematologic grade 3 or 4 toxicities was similar in both arms.
One question not addressed in these trials is whether patients with certain subtypes of ovarian cancer might have better outcome if treated with WAR instead of chemotherapy. A retrospective study of patients with predominantly early-stage clear cell carcinomas treated in Okinawa from 1996 to 2004 found superior 5-year overall survival (82%) and disease-free survival (82%) compared to patients treated with platinum-based chemotherapy (rates of 33% and 25%, respectively), although interpretation is difficult to small patient numbers and the possibility of selection bias.79
In summary, these randomized trials generally found WAR or IP 32P was less effective or more toxic than platinum-containing regimens. Hence, adjuvant radiation therapy has fallen out of use as the primary treatment for patients with high-risk, early stage ovarian cancer.
Management of Advanced-Stage Disease
Debulking Surgery
The theoretical benefits of debulking surgery include removal of large, necrotic tumors with poor blood supply that might lead to impaired chemotherapy delivery. It has also been postulated that tumor debulking may permit residual tumor to proliferate more rapidly and thereby enhance sensitivity to postoperative chemotherapy. Although neither of these mechanisms has been proven, the association between successful surgical cytoreduction and more favorable outcome has been demonstrated in many surgical series.14,55,63
The definition of optimal cytoreduction achieved through debulking surgery has varied through the years, but residual disease of ≤1 cm in maximum individual tumor diameter is most widely accepted. Recently, however, there has been a trend toward redefining optimal cytoreduction to refer more specifically to those patients who have no gross residual disease, reflecting complete cytoreduction to microscopic residual status.80
Primary debulking surgery refers to performance of surgery prior to administration of first-line chemotherapy and is the standard approach for managing most patients with suspected epithelial ovarian cancer. Despite the importance of primary debulking surgery, some patients with advanced disease are not ideal surgical candidates due to a poor performance status, or extensive amounts of bulky intra-abdominal disease as assessed by radiographic studies, and therefore may not be appropriate candidates for this procedure. In these cases, initiating neoadjuvant chemotherapy is a reasonable approach, followed by an interval attempt at surgical cytoreduction in responding patients after three cycles of chemotherapy have been administered. In this instance, surgery is referred to as interval debulking surgery.81,82 Theoretical advantages of neoadjuvant chemotherapy in this setting are a more rapid improvement in quality of life, and, if interval debulking surgery is ultimately performed, a technically more feasible operation with shorter hospitalization and less morbidity. Prospective randomized trials incorporating neoadjuvant chemotherapy in the management of advanced-stage ovarian cancer are under way to better define the value of this strategy. The first of these to be reported was conducted by the European Organisation for Research and Treatment of Cancer (EORTC)-National Cancer Institute of Canada (NCIC), involving 632 patients who were randomized to receive either primary debulking surgery followed by six cycles of platinum-based chemotherapy, or neoadjuvant chemotherapy for three cycles, followed by interval debulking surgery in responding or stable patients, followed by an additional three cycles of chemotherapy.83 Eligibility for this study included stage IIIC and IV disease, performance status ≤2, and no disease in the upper abdomen measuring <2 cm in greatest diameter as assessed by CT or laparoscopy. Patients with retroperitoneal lymph node involvement as their only site of stage III disease were not included.84 In addition, confirmation of the clinical impression of ovarian cancer was required prior to instituting neoadjuvant therapy, using either core biopsy or fine needle aspiration, with supportive serology including a CA 125:CEA ratio of ≥25. For patients whose CA 125:CEA ratio was <25, appropriate studies were performed to exclude the possibility of a breast or gastrointestinal primary. The results of this trial show that the overall survival was equivalent between the two treatment approaches, despite the fact that the rate of optimal cytoreduction was higher in patients undergoing neoadjuvant chemotherapy. There was a trend toward less postoperative morbidity in those who received neoadjuvant therapy, although this did not reach statistical significance. However, for the subset of patients whose disease was <5 cm in greatest diameter at study entry, their overall survival was improved with primary cytoreduction compared to neoadjuvant therapy.84 Based upon the results of this study, the approach of neoadjuvant therapy and interval debulking surgery is a reasonable option for patients with bulky upper abdominal peritoneal disease >5 cm in diameter, or those with medical comorbidities that preclude safe performance of primary surgical cytoreduction. Preliminary results from a second randomized trial of neoadjuvant chemotherapy and interval debulking surgery being performed in the United Kingdom are supportive of this approach, and we await the results of a third confirmatory trial being performed by the Japanese Clinical Oncology Group.85 Despite the value of neoadjuvant chemotherapy and interval debulking surgery in appropriately selected patients, primary cytoreduction should still be the preferred initial option for patients with stage IIIC and IV disease with good performance status who have <5 cm upper abdominal disease, who have retroperitoneal only disease preoperatively, or who might otherwise be considered ideal candidates for IP therapy if optimally cytoreduced (see the following).
Postoperative Chemotherapy for Epithelial Ovarian Cancer
Advanced-stage epithelial ovarian cancer is a chemoresponsive disease in the majority of cases, although relapse often occurs and resistance eventually develops to most forms of treatment. The platinum compounds remain the single most active drugs in the treatment of this disease. Cisplatin was the most frequently used platinum compound in the late 1970s and early 1980s, although it was soon recognized that the use of carboplatin instead of cisplatin conferred an equivalent survival advantage, but with less neuropathy, nephropathy, and emesis.86
Although numerous combination chemotherapy regimens have been studied over the past three decades, the combination of an IV platinum compound and a taxane such as paclitaxel is now accepted as an appropriate first-line, postoperative option for many patients. The response rate of this combination is as high as 70% for patients with suboptimally debulked disease, and over 80% for patients who are optimally cytoreduced.61,62 Platinum agents exert their effects by forming intrastrand DNA adducts with guanosine bases, whereas taxanes mediate cytotoxicity by binding to and stabilizing the tubulin polymer, thereby preventing physiologic dissociation of the mitotic spindle at the completion of M phase. Given these nonoverlapping mechanisms of action, it was not surprising to find that taxanes were active agents in the treatment of some patients with relapsed, platinum-resistant ovarian cancer.87 These observations formed the basis for investigating the platinum and taxane combination in the first-line setting.
Two phase 3 randomized trials begun in the early 1990s compared a cisplatin and paclitaxel combination to a cisplatin and cyclophosphamide regimen (Table 76.4). In the GOG study, patients with suboptimally resected stage III or IV ovarian cancer were randomized to receive six cycles of either cisplatin and cyclophosphamide or the experimental arm of cisplatin and paclitaxel.62 Women treated with the paclitaxel-containing program experienced a statistically significant improvement in response rate (73% versus 60%; p <0.01), median PFS (18 months versus 13 months; p <0.001), and median overall survival (38 months versus 24 months; p <0.001).
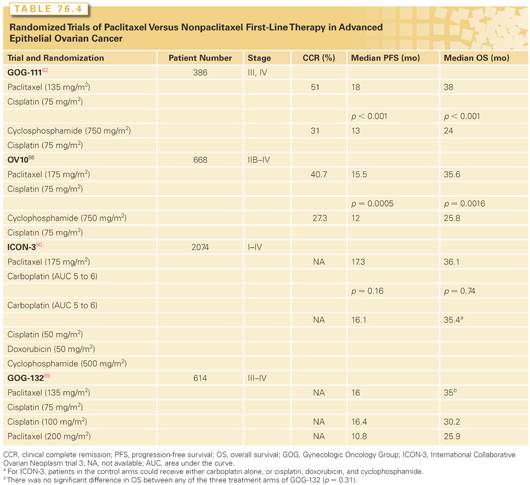
These results were confirmed in a trial conducted in Europe and Canada that used a similar but not identical study design.88 In this trial, paclitaxel was delivered as a 3-hour infusion of 175 mg/m2, whereas in the GOG study a dose of 135 mg/m2 was administered over 24 hours. In addition, a much larger percentage of patients in the control arm of the European-Canadian study received paclitaxel as second-line therapy after relapse, compared to the control arm of the GOG trial. Despite these differences, this study also revealed a statistically significant improvement in both PFS and overall survival associated with the paclitaxel-containing regimen (see Table 76.4).
Neither the GOG nor the European-Canadian trials was able to address whether the survival benefit observed with the platinum-taxane doublet was related to the use of combination chemotherapy or whether it could also be achieved through the use of sequential monotherapy. In an attempt to address this issue, the GOG conducted a randomized trial in which patients with suboptimally debulked advanced disease received either single-agent cisplatin (100 mg/m2), single-agent paclitaxel (200 mg/m2 administered over 24 hours), or the combination of cisplatin (75 mg/m2) and paclitaxel (135 mg/m2 administered over 24 hours).89 Survival was similar between the three study arms, despite a superior objective response rate to both platinum-containing regimens (67%), compared to single-agent paclitaxel (42%). This result is likely explained by the fact that approximately 50% of patients in the single-agent arms were switched to the alternative agent before documented disease progression. These results support the concept that sequential treatment with a platinum agent, followed by paclitaxel, may be therapeutically equivalent to combination therapy with both agents in advanced ovarian cancer. However, as crossover to the alternative agent was not a formal part of this study’s design, this interpretation should be viewed with caution, and combination first-line chemotherapy with a taxane and platinum compound remains the standard of care.
A third European randomized trial (ICON-3) compared a nonpaclitaxel, platinum-based control regimen (either single-agent carboplatin or the combination of cisplatin, doxorubicin, and cyclophosphamide) to an experimental arm of carboplatin and paclitaxel.90 Surprisingly, this study failed to reveal a difference in survival between either control arm and the paclitaxel-containing experimental arm. Perhaps the most likely explanation for this observation is that one-third of the patients in the control arms of ICON-3 ultimately received paclitaxel at some point in their disease course.91 Thus, as in the previously mentioned three-arm GOG study,89 this outcome provides circumstantial evidence to suggest that the sequential administration of these two active agents may be therapeutically equivalent to combination drug delivery in this setting.
Three randomized trials have directly compared a carboplatin and paclitaxel combination to cisplatin and paclitaxel (Table 76.5).61,92,93 These studies found equivalent PFS and overall survivals for either carboplatin- or cisplatin-containing regimens, but with a more favorable toxicity profile associated with carboplatin-based treatment. Thus, the carboplatin-based combination (carboplatin, area under the curve [AUC] = 5 to 6, and paclitaxel, 175 mg/m2, administered over 3 hours) is preferred when systemic chemotherapy is indicated, due to reduced toxicity and the ability to give paclitaxel over a shorter infusion time. For individuals who may have difficulty tolerating a combination regimen (e.g., those with marginal performance status or significant comorbid medical conditions), it is reasonable to initiate treatment with IV single-agent carboplatin and later add IV paclitaxel to the regimen or deliver the drugs as sequential single agents. For appropriate patients with stage III disease who are optimally cytoreduced, IP chemotherapy is an important new option, which will be described in detail subsequently.

The optimal choice of taxane has also been investigated in the first-line setting. A randomized trial comparing IV carboplatin (AUC = 5) plus paclitaxel (175 mg/m2) to IV carboplatin (AUC = 5) and docetaxel (75 mg/m2) has shown equivalent response rates and PFS and overall survival for the two programs, although their toxicity profiles differed.94 More grade 4 neutropenia occurred with the docetaxel-containing regimen, and a greater incidence of grade 2 or 3 neuropathy was observed with the paclitaxel-containing program. These data indicate that a carboplatin and docetaxel combination is an acceptable first-line regimen for patients with advanced ovarian cancer, especially in the setting of preexisting neuropathy (where paclitaxel may be difficult to tolerate).
There appears to be no value in extending platinum-based first-line therapy beyond six cycles.95,96 Furthermore, there is no convincing evidence to suggest a benefit to the addition of cytotoxic drugs such as liposomal doxorubicin, epirubicin, topotecan, or gemcitabine to the platinum and taxane doublet.97 A recent randomized trial performed by the Japanese GOG demonstrated a PFS and overall survival advantage for the use of weekly paclitaxel (in conjunction with day 1 carboplatin) in newly diagnosed patients with ovarian cancer, and confirmatory trials are under way.98 Given the activity of bevacizumab in relapsed disease, there is interest in investigating the value of this agent in the first line and maintenance setting (see the following).
Intraperitoneal Chemotherapy
Epithelial ovarian cancer is largely confined to the peritoneal space during most of its natural history.2 Given this relatively localized distribution, efforts to instill chemotherapy directly into the peritoneal cavity have received a great deal of attention over the past two decades. The rationale for this approach is based on the observation that many active drugs such as cisplatin and paclitaxel have favorable peritoneal-to-plasma concentrations, on the order of 20 to 1 and 1,000 to 1, respectively.99 The ability to deliver high local concentrations of active drugs, with generally acceptable systemic side effects, suggested that it may be possible to achieve more effective cytoreduction of disease that is present at the peritoneal surface. Given the rather limited penetration of such drugs into peritoneal tumor, to a depth of only a few millimeters, patients with optimally cytoreduced disease are theoretically most likely to benefit from this approach.
Three randomized trials have addressed the role of IP chemotherapy in the first-line management of patients with optimally debulked stage III disease (Table 76.6). Two of these trials showed a statistically significant improvement in both PFS and overall survival rates for patients treated with IP chemotherapy, and the other demonstrated improvement in PFS only (although a nonsignificant trend for improved overall survival in the IP arm was noted). The first of these was an intergroup trial performed in 546 optimally debulked stage III patients with ≤2-cm residual disease (GOG 104).100 Note that this definition of “optimally debulked” was widely accepted at the time, although subsequently a 1-cm cutoff has been adopted. Patients were randomized to receive either IP cisplatin or IV cisplatin, in combination with IV cyclophosphamide, every 3 weeks for six cycles (note that paclitaxel was not an approved drug at the time of this study’s design). The median survival of all eligible patients was 49 months for the IP cisplatin arm compared to 41 months for the IV cisplatin group (p = 0.02; HR = 0.76). There was also a trend toward a higher likelihood of pathologic complete remission in the IP arm compared to IV treatment (47% versus 36%, respectively). However, the positive results of this study were overshadowed by the emerging value of the IV paclitaxel and platinum combination in the first-line setting,62 and the added benefit of the IP route remained uncertain. Specifically, it was argued that perhaps the use of IV paclitaxel might negate the benefit of IP therapy, and this issue led to a second randomized IP trial that included paclitaxel.

The second study performed by the GOG (GOG 114) involved optimally debulked patients with stage III disease (≤1-cm diameter residual) who were randomized to receive either IV paclitaxel (135 mg/m2 over 24 hours) and IV cisplatin (75 mg/m2) for six cycles, or IV carboplatin (AUC = 9) for two cycles followed by IV paclitaxel (135 mg/m2 administered over 24 hours) and IP cisplatin (100 mg/m2) for six cycles.101 The median PFSs for the IP and IV arms were 28 months and 22 months, respectively (p = 0.01; one-sided t test). However, there was only a borderline significant trend in overall survival in favor of the IP treatment arm (63.2 months versus 52.5 months, respectively; p = 0.05, one-sided t test), but with substantial toxicity partly due to the use of high-dose, single-agent carboplatin in the experimental arm. The significant toxicity of this regimen, coupled with only a borderline significant survival difference, did not provide convincing enough evidence to adopt this regimen for widespread use.
The third GOG study (GOG 172) eliminated the use of high-dose carboplatin and introduced a second dose of paclitaxel, administered via the IP route, on day 8.102 Patients with optimally debulked disease (≤1-cm diameter residual) were randomized to receive either a control arm of IV paclitaxel (135 mg/m2 administered over 24 hours) and IV cisplatin (75 mg/m2) for six cycles, or IV paclitaxel on day 1 (135 mg/m2 administered over 24 hours), IP cisplatin on day 2 (100 mg/m2), and IP paclitaxel on day 8 (60 mg/m2
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








