(1)
Medical Sciences Division Northern Ontario School of Medicine West Campus, Lakehead University, Thunder Bay, Ontario, Canada
Key Topics
Molecular pathology of ovarian cancer (OvCa).
Circulating OvCa biomarkers.
Circulating OvCa miRNA biomarkers.
Circulating OvCa protein biomarkers.
Circulating OvCa cells.
Key Points
OvCa is mostly a disease of postmenopausal women, but as many as 30 % of cases occur in women under the age of 50. The incidence is on the rise; however, tumors detected early have good prognosis. Noninvasive biomarkers for early detection of OvCa in perimenopausal and postmenopausal women should improve the outlook for OvCa.
Serum CA125 remains a useful biomarker of OvCa. Its utility in multivariate index assays increases the accuracy of OvCa risk prediction.
Novel circulating OvCa biomarkers, including miRNAs, proteomic discoveries, as well as circulating OvCa cells add to the clinical armamentarium of OvCa biomarkers.
13.1 Introduction
Globally, over 239,000 women were estimated to be diagnosed with OvCa in 2012, while in the same year as many as 140,000 women died from the disease. This mortality rate is on the rise, because in 2010, 160,000 women died from OvCa, which was up from 113,000 in 1990. OvCa is the most lethal gynecologic cancer, and globally is the 7th cause of cancer-related deaths. In the US alone, the 2016 estimated cases and deaths are 22,280 and 14,240 respectively. There are geographic variations in the incident rates, with rates being higher in the more developed than the less developed parts of the world. For example, the rate of 11.7/100,000 women in the UK contrasts sharply with that of 4.1/100,000 for other parts of the world.
The 5-year survival rate for women diagnosed with OvCa has increased over the past decades due to improved gynecologic surgical techniques, coupled with combination therapies, but not due to early detection. While it is established that early detection of organ-confined tumors (stage I) has the best prognosis, OvCas are rarely detected early. Indeed, only ~15 % are localized cancers, with the vast majority having some regional spread (18 %) or worse still distant metastasis (~60 %). The survival rate can be as high as 92 % for organ-confined early-stage disease. This survival rate drops significantly to under 30 %, when cancers spread to the peritoneum or distant organs. The need for early detection biomarkers is thus obvious. Because there are no recommended screening procedures, coupled with the need for early and appropriate treatment, the “risk malignancy index” (RMI) was established in the early 90s. This risk index includes ultrasound detection of pelvic masses, elevated serum CA125, and menopausal status. While RMI has a good sensitivity (71 %–88 %) and a high specificity (74 %–97 %), only women considered to be above average risk receive such periodic screening, leaving many, especially premenopausal women without any screening, though OvCa occurs in ~30% of this population. Indeed, while commonly diagnosed in women above the age of 55, the incidence rises from about age 35–39.
CA125 is the most commonly used noninvasive screening biomarker. Overall, CA125 is elevated in about 80% of women with OvCa. This rate is ~60% in stage I and up to 90% in advanced stage III/IV diseases. Thus, its use in early detection has shortcomings. However, because it has established utility in monitoring chemotherapy response, the FDA has approved this biomarker for such intended use. However, the CA125 assay has limitations. Endometriosis, menstruation, and benign uterine lesions in premenopausal women can elevate circulating levels of CA125. There is still therefore a need for better noninvasive biomarkers with increased accuracy and companion diagnostic ability for OvCa.
13.2 Screening Recommendations for OvCa
There are currently no screening programs or recommendations for OvCa detection in the at-risk population. In women with established family history of the disease, periodic CA125 measurements coupled with transvaginal ultrasound are recommended. Current utility and benefits of CA125 biomarker in OvCa include:
Monitoring for disease recurrence after treatment. Elevated levels indicate recurrence in up to 70 % of cases.
Monitoring for effectiveness of chemotherapy. In 90 % of cases, persistent elevated levels are indicative of disease presence. Response should be associated with a decline.
Used to assess disease burden with over 90 % accuracy.
The NACB also recommends CA125 measurements for:
Differential diagnosis of pelvic masses.
Early detection of cancer in combination with transvaginal ultrasound in people with familial predisposition.
Detection of recurrence.
Monitoring therapy response.
Prognosis.
Other preventive measures of OvCa are the provision of prophylactic surgery to BRCA1 and BRAC2 carriers and kindred of Lynch syndrome who are at high risk for the disease. While the vast majority (~75 %) of OvCas are diagnosed in postmenopausal women, as many as 30 % of tumors are detected in people under the age of 55. It would therefore be helpful to develop simple cost-effective tests that can be used to screen all women after the age of 40.
13.3 Molecular Pathology of OvCa
13.3.1 Origins of OvCa
OvCas are from diverse cellular origins; however, the most common ovarian tumors are of epithelial origin (EOCs). These tumors are classified histologically based on the WHO/FIGO recommendations into the following different types: serous, mucinous, endometrioid, clear cell, and transitional types. The majority of EOC are of serous histology. The different types harbor shared and unique molecular changes, and also have different clinical behaviors. Epithelial OvCas are also heterogeneous in regard to their origins, which is reminiscent of the anatomic relationship and location of the ovaries. The putative precursor lesions of surface EOCs are:
In situ lesions of the surface epithelium or surface epithelial inclusion glands.
Benign epithelial tumors.
Endometriosis.
However, the most common subtype of OvCa, high-grade serous carcinoma (HGSC), is mostly of extra-ovarian source. Various molecular genetic data suggest the majority (~60 %) of HGSCs develop from intraepithelial lesions in the fallopian tube, and involve the ovaries secondarily. Additionally, some HGSCs may arise from cortical inclusion cysts. It is thus becoming evident that the majority of OvCas originate from outside the ovaries.
13.3.2 Dualistic Molecular Subtypes of OvCa
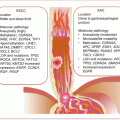
Irrespective of their origins, from actionable clinical perspective, a dualistic model recognizes low-grade (type I) and high-grade (type II) EOCs. These two have different histologic grades and types of molecular events (Fig. 13.1).
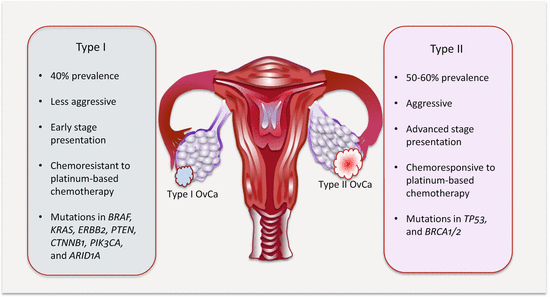
Type I are mostly low-grade serous EOCs. They are indolent tumors that present early (at stage I). They develop in a stepwise predictive fashion from atypical proliferative or borderline epithelial tumors. They also include mucinous, endometrioid, clear cell, and malignant Brenner tumors. Molecularly, these tumors are more genetically stable and rarely harbor TP53 mutations. They mostly activate the MAPK, PI3K, and WNT/β-catenin signaling pathways due to mutations in BRAF, KRAS, and ERBB2 (serous); KRAS (mucinous); PTEN and CTNNB1 (endometrioid); and PIK3CA (clear cell).
Type II EOCs are high-grade serous carcinomas. These are aggressive tumors that present at advanced stage. They are commonly associated with chromosomal aberrations and TP53 mutations. Additionally, BRCA mutations are associated with the development of these fallopian tube-derived HGSCs. Other aggressive OvCas are malignant mixed mesodermal tumors (carcinosarcomas) and undifferentiated carcinomas.

Fig. 13.1
Features of the two major subtypes of OvCa
This histologic delineation is important for OvCa biomarker exploration, disease prevention, screening, and treatment approaches.
13.4 Circulating OvCa Biomarkers
Notwithstanding its limitations, CA125 remains a useful noninvasive biomarker of OvCa. To improve upon OvCa management, efforts at uncovering novel biomarkers including ccfDNA levels, as well as genetic and epigenetic alterations in ctDNA are sought after. Additionally, alterations in the levels of miRNA and novel proteins are pursued. An emerging class of prognostic biomarkers is circulating OvCa cells.
13.4.1 Circulating Cell-Free Nucleic Acid Content as OvCa Biomarkers
The promising role of ccfDNA in the diagnosis, treatment prediction, and prognosis of women with OvCa has been demonstrated by a number of studies. In a proof of principle study, female nude mice were injected intraperitoneally with OvCa cell line, HeyA8, and it was demonstrated that ccfDNA levels increased with increasing tumor burden, and also modulated with chemotherapy. Thus, tumor ccfDNA was elevated at 63 % above baseline in 24 h following docetaxel therapy, and then declined to below 20 % of baseline in 72 h, with further decline to below 83 % of baseline in 10 days after therapy [1]. In a follow-up study, ccfDNA was quantified in women with OvCa and healthy controls targeting GAPDH, ACTB, and HBB [2]. In this cohort of women with advanced stage OvCa (stage III/IV), ccfDNA was significantly higher than in healthy control women at all three genomic loci examined. Zachariah et al. also demonstrated the potential diagnostic utility of ccfDNA in women with OvCa [3]. They revealed that ccfDNA from both nuclear and mitochondrial genomes were significantly increased in cancer patients compared to both healthy controls and women with benign ovarian diseases. Additionally, there were significant differences in the levels of mtDNA between women with OvCa and those with endometriosis. CcfDNA has roles in predicting outcomes in OvCa women on therapy. Wimberger et al. revealed that residual tumor load in OvCa patients on platinum-based chemotherapy contributes to increases in ccfDNA [4]. There was also a significant association between residual tumor volume (>1 cm) after surgery and the levels of serum DNA. The increases in ccfDNA as well as the presence of DTCs were significantly associated with increased relapse and poor overall survival. In multi-resistant OvCa women on bevacizumab-targeted therapy, ccfDNA significantly correlated with PFS (p = 0.0004) and OS (0.005) in both univariate and multivariate analyses. High ccfDNA was a positive predictor of poor prognosis [5]. Circulating cfDNA, p53 antibodies, and KRAS mutations could predict the outcomes in women with different types of OvCa. Circulating antibodies against p53 and ccfDNA were frequently associated with women who had HGSCs, and this predicted worse overall survival. Similarly, circulating KRAS mutations were common in women with mucinous OvCa and was an indicator of worse OS [6].
13.4.2 Circulating OvCa Epigenetic Biomarkers
13.4.2.1 Circulating OvCa Diagnostic Epigenetic Biomarkers
Methylated genes are explored as circulating diagnostic biomarkers of OvCa. Ibanez et al. screened ovarian tumors and matched preoperative sera/plasma and peritoneal fluids for RASSF1A and BRAC1 methylation status [7]. Hypermethylation of one or both genes was demonstrated in 68 % of tumors. They then expanded coverage by including the following genes: APC, CDKN2A, DAPK, and CDKN2A/ARF, which enabled the remaining tumors to be identified. Identical methylation pattern was present in 82 % of serum/plasma samples (including 76.5 % of stage I disease samples). Peritoneal fluid was positive in 93.3 % of the samples, including samples from patients with tumors that were scored conventionally as atypia or negative by cytology. Microarray-based screening of 58 genes, and using the most differentially methylated genes in naïve Bayesian analysis enabled 10 genes to be identified for further study. The sensitivity and specificity in tissue samples were 69 % and 70 %, respectively. A 5-gene panel analyzed in plasma samples achieved a sensitivity of 85 % and specificity of 61 %. This assay demonstrates diagnostic potential [8]. In a follow-up study, Levenson’s group expanded their proof-of-principle study in an attempt to differentiate women with healthy ovaries from those with OvCa and benign ovarian diseases, and to possibly differentiate benign ovarian diseases from cancer using methylation of a number of gene sets in ccfDNA. Methylation of RASSF1A, CALCA, and EP300 differentiated cancer from healthy controls at a sensitivity of 90% and specificity of 86.7 %. Similarly, methylation of BRAC1, CALCA, and CDKN1C differentiated women with benign ovarian disease from healthy controls at a sensitivity of 90 % and specificity of 76.7 %. Finally, RASSF1A and PGR-PROX methylation in ccfDNA differentiated cancer from benign ovarian disease [9]. Bondurant et al. used a sensitive assay that enabled detection of 1 methylated among 100,000 unmethylated alleles [10]. Using this assay, RASSF1A was methylated in 51 % of invasive serous OvCa samples, and was concordant in all available matched preoperative serum samples. Serial serum sampling enabled therapy response monitoring that was detectable as fluctuations in RASSF1A methylation in some patients, reflective of disease status.
13.4.2.2 Circulating OvCa Prognostic Epigenetic Biomarkers
In addition to tumor grade and histologic cell type, the cytologic examination of peritoneal fluid is part of the staging strategies of OvCa. Women with positive peritoneal fluid cytology have poor prognosis regardless of FIGO stage. The inherent issues with cytology call for better prognostic biomarkers or complementary assays to cytology. As a proof-of-principle study to identify sensitive prognostic methylation biomarkers, peritoneal fluids were collected at surgery from women with OvCa, who also received platinum-based chemotherapy after surgery. Fifteen genes were selected for study. Women with fewer methylated genes had poor or shorter overall survival evidenced by univariate and multivariate analysis, independent of age, FIGO stage, or grade. Thus, methylation in genes from this series may confer sensitivity to platinum-based chemotherapy [11].
13.4.3 Circulating OvCa Genetic Biomarkers
Studies of genetic changes in circulation of OvCa patients have mainly been proof of concept discovery studies. Serum and peritoneal fluid DNA samples from OvCa patients were analyzed for known tissue-specific genetic changes. Six polymorphic markers on four chromosomes were assessed for LOH detection, which were positive in 85 % of serum and 63 % of peritoneal fluid samples. Half of the LOH observed in tissue samples were specifically detected in sera [12]. Zhang et al. questioned the relevance of LOH on chromosome 3p14 in serum DNA samples from patients with OvCa. Four polymorphic markers (D3S1029, D3S1228, D3S1300, D3S1481) were employed, and the detection frequency of at least one alteration was 78 %. Indeed, 45 % of serum samples had more than a single MSA. LOH was associated with advanced stage disease [13]. LOH on chromosomes 13q, 17q, 17p, and 22q, and mutations of TP53 and KRAS in tissue and peritoneal washes from patients with OvCa revealed the presence of tissue LOH in 38 % of peritoneal fluids. TP53 mutations were in 21 % of tissue samples and all (100 %) were detectable in peritoneal fluid. In total, 57 % had genetic changes (all serous adenocarcinomas), and these changes were detectable in 62 % of matched peritoneal fluid. As a proof of principle, this has implications for OvCa early detection [14]. TP53 mutations occur in up to 81 % of OvCas, and plasma TP53 exon 5–8 mutations were detected in 16.7 % of preoperative patients [15]. Thus, genetic alterations from OvCa are concentrated more in peritoneal than circulating fluids.
13.4.4 Circulating OvCa Noncoding RNA Biomarkers
13.4.4.1 Deregulated miRNAs in OvCa Tissues and Cell Lines
MiRNA deregulation is associated with OvCa. In tissues, and also in body fluids, the aberrant expression of let-7 and miR-200 family members appears to be associated with various types of OvCa. Several miRNAs, possibly with tumor suppressor functions, are downregulated in OvCa tissues and cell lines. These include let-7d, miR-125b-1, miR-127, miR-140, miR-145, and miR-199a. Specifically, in serous OvCa, let-7b, miR-10b, miR-26a, miR-29a, miR-99a, miR-100, miR-125a, miR-125b, miR-143, miR-145, miR-199a, and miR-214 are downregulated. Similarly, a number of possible oncomirs are upregulated in OvCa samples, and some are subtype associated. OvCa subtype-specific upregulated miRNAs include miR-30a-5p and miR-30a-3p in clear cell OvCa and miR-192 and miR-194 in mucinous OvCa, while serous OvCa is associated with increases in miR-16, miR-20a, miR-21, miR-23a, miR-23b, miR-27, miR-93, miR-141, miR-200a, miR-200b, and miR-200c. Some miRNAs deregulated in epithelial OvCa tissue samples also appear to correlate with various prognostic parameters such as DFS, PFS, Recurrence-FS, and OS. Those associated with good prognosis include miR-100, miR-150, miR-187, miR-200a, miR-200c, miR-335, miR-410, miR-645, and the ratio of miR-221/miR-222, while deregulated miR-21, miR-25, miR-29, miR-203, and miR-221 confer poor prognosis.
Several OvCa-associated miRNAs have defined functions in tumor initiation and progression and serve as therapeutic targets as well. Some miRNAs are implicated in EMT, cancer stem cell maintenance, angiogenesis, and extracellular matrix remodeling in OvCa. As examples, the tumor suppressor miR-138 represses genes involved in EMT and hence is downregulated in metastatic OvCa cells. Similarly, miR-125a inhibits EMT through AT-rich interactive domain 3B (ARID3B). EGFR signaling through the ETS family of transcription factor (PEA3) represses miR-125a expression in metastatic OvCa. MiR-200a and miR-200c are also downregulated in CD117-positive/CD44-positive OvCa stem cells compared to CD117-negative/CD44-negative cells. Increased expression of these miRNAs decreased ZEB1 and vimentin levels in association with E-cadherin expression, which impaired migration, invasion, and colony formation. MiR-125b, miR-145, and miR-199a target HIF-1α and VEGF to suppress tumor angiogenesis, and all are deregulated in OvCa. Finally, miR-335 is downregulated in OvCa, because it inhibits metastasis by targeting the glycoprotein tenascin C (TNC) that is a negative regulator of cell–extracellular matrix interaction.
13.4.4.2 Circulating OvCa miRNA Biomarkers
Circulating miRNA deregulation with diagnostic and prognostic potential are demonstrated in samples from OvCa patients. While mostly not validated, their potential role in the clinic is expected and should make a difference in the diagnosis and management of this disease.
In 2008, Taylor et al. first reported on differential levels of exosomal miRNA between women with OvCa and those with benign ovarian adenomas [16]. They selected eight known OvCa-associated miRNAs (miR-21, miR-141, miR-200a, miR-200b, miR-200c, miR-203, miR-205, and miR-214) and demonstrated that these were significantly enriched in exosomes from cancer patients compared to controls. Of interest, these circulating exosomal miRNA levels were not correlated with tumor stage or grade, suggesting their potential as early OvCa detection biomarkers. The elevated levels of the miR-200 family members (miR-200a, miR-200b, and miR-200c) in sera from women with serous OvCa were confirmed. Resnick et al. reported a similar study, whereby 21 differentially expressed miRNAs in OvCa tissue samples were selected a priori, and tested on serum samples from patients and controls [17]. Of the 21 miRNAs, five (miR21, miR-29a, miR-92, miR-93, and miR-126) were elevated, while the levels of three (miR-99a, miR-127, and miR-155) were decreased in sera from women with OvCa compared to healthy controls. Using whole blood samples from patients and controls, eight miRNAs (miR-16, miR-29a, miR-30c-1, miR-106b, miR-155, miR-146a, miR-191, and miR-383) were demonstrated to be deregulated in cancer patients, and this finding was consistent with those in OvCa tissue samples. miR-30c-1-3p was upregulated, while downregulated were miR-181a-3p, miR-342-3p, and miR-450b-5p [18]. A microarray profiling of sera, ascites, and tissue samples from women with serous OvCa uncovered four miRNAs (let-7b, miR26a, miR-132, and miR-145) that were significantly decreased in sera from patients compared to controls [19]. Plasma let-7f and miR-205 have also been identified as early detection biomarkers (stage I) of OvCa [20].
Endometriosis is a precursor lesion to some types of OvCa. The ability to differentiate endometriosis from OvCa is clinically relevant. The work by Suryawanski et al. sheds some light on the potential of miRNA in this regard [21]. Global profiling of circulating miRNA in women with endometriosis-associated OvCa enabled the identification of miR-16, miR-191, and miR-195 to be elevated in women with endometriosis. This miRNA signature had a detection sensitivity of 88 % and specificity of 60 %. Three other miRNAs (miR-16, miR-21, and miR-191) were able to differentiate women with endometriosis-associated OvCa from healthy women at a sensitivity of 86 % and specificity of 85 %. Importantly, miR-21, miR-362-5p, and miR-1274a performed at a sensitivity of 57 % and a specificity of 91 % in separating between women with endometriosis and those with endometriosis-associated OvCa. Moreover, miR-21, miR-191, and miR-1975 could stratify women with sOvCa and endometriosis-associated OvCa at a sensitivity of 86 % and specificity of 79 %. A separate set of miRNAs (miR-362-5p, miR-628-3p, and miR-1915) performed at a sensitivity of 90 % and specificity of 73 % in differentiating women with endometriosis from those with sOvCa. Finally, for separating between women with sOvCa and healthy controls, miR-16, miR-191, and miR-4284 achieved a sensitivity and specificity of 90 % and 55 %, respectively.
Several circulating miRNAs that are deregulated in OvCa have been associated with clinical outcomes. Decreases in let-7f levels correlate with poor prognosis, and elevated miR-221 in sera from women with eOvCa is associated with FIGO stage and tumor grade. In multivariate analysis, high circulating miR-221 was an independent predictor of poor prognosis [22]. Circulating levels of miR-92 correlate with lymph node metastasis and clinical stage of OvCa, while advanced FIGO stage, high tumor grade, and poor OS were associated with elevated miR-21 [23]. Five preoperative plasma miRNAs significantly predicted shorter OS. However, after multiple testing, only miR-1290 emerged as a robust prognostic biomarker, with AUROCC of 0.87, p = 0.05 [24].
13.4.5 Circulating OvCa Proteomic Biomarkers
Proteomic approaches have made important contributions in the identification of biomarkers for OvCa management. Still remaining as an important issue is the discovery of screening biomarkers. Because of the low disease prevalence, a sensitivity of >75 % and a specificity of >99.6 % are required to achieve an acceptable PPV of 10 %. Thus, there have been several proteomic biomarker discovery research efforts to meet these demanding performances.
13.4.5.1 Serum Proteomic Spectral Peaks as OvCa Biomarkers
Several discovery studies have explored the use of serum m/z spectral peak signatures for OvCa detection. The seminal work of Petricoin et al. is noteworthy [25]. This group used serum proteomic SELDI TOF MS spectra applied to an iterative search algorithm to identify a discriminatory pattern for cancer detection. This “cluster -proteomic -pattern” biomarker achieved a sensitivity of 100 %, a specificity of 95 %, and a PPV of 94 % for OvCa. Since then, several groups have applied proteomic spectral patterns as cancer biomarkers.
Four peptide peaks at m/z 6195, 6311, 6366, and 11498 achieved a sensitivity of 87 % and a specificity of 95 % in a validation sample set. A peak at m/z 4475 only appeared after chemotherapy [26]. Lin et al. observed four peaks (m/z 5147.06, 6190.48, 11522.6, 11537.7 Da) in plasma from cancer patients but not controls, and 2 peaks (5295.5, 8780.48) in controls but not in cancer patients [27]. The sensitivity and specificity of these peaks were 96.3 % and 100 % for OvCa. Wang et al. also observed seven discriminatory peaks at m/z 3940, 4099, 4144, 4479, and 5488 (upregulated) and 8588 and 13783 (downregulated) in advanced stage OvCa that had sensitivity of 93.94 % and specificity of 93.55 % [28]. These biomarkers also detected 90 % of early stage OvCas. Of 98 peaks that discriminated malignant from benign tumors, 46 were finally identified from average linkage clustering to be more relevant [29]. Similarly, in women with normal CA125 levels, 25 peaks discriminated malignant from the benign group [30]. Mass spectrometric peaks of sera could differentiate stage I/II disease from controls at a sensitivity of 80% with AUROCC of 0.82, and stage III/IV at a sensitivity of 93 % and AUROCC of 0.92. Note that the majority of these studies involved small samples sizes and hence are only proof-of-concept discovery findings.
13.4.5.2 Serum OvCa Proteomic Biomarkers
Proteomics approaches have enabled the identification of several proteins and peptides with differential levels in circulation of women with OvCa. Among these numerous proteins are haptoglobin, apolipoprotein A1, transthyretin, amyloid AI, transferrin, β-hemoglobin, and β2-microglobulin that show potential as OvCa biomarkers.
Serum amyloid A1 and hemoglobin are among the early protein biomarkers identified by proteomic studies for OvCa. Moshkovskii et al. observed invariant peaks at m/z 11.7 and 11.5 kDa that were present in 55.6 % of OvCa but at low intensities in only 5.8 % of controls [31]. These peaks were identified as amyloid A1 (11.68 kDa) and its N-terminal arginine-truncated form (11.52 kDa). Woong-Shick et al. also identified two peaks with significant differential expression between cancer and controls as hemoglobin-α (15.1 kDa) and hemoglobin-β (15.8 kDa) chains [32]. The sensitivity for intact hemoglobin was 77 % in sera from cancer compared to controls.
Several investigators have demonstrated the clinical performance of serum apolipoprotein A1 in OvCa. Kozak et al. used SELDI TOF MS to identify 14 differentially expressed peaks categorized into three protein panels designated as screening panel (five candidates), validation panel I (five candidates), and validation panel II (four candidates) [33]. Independently, they all performed very well in differentiating between benign and malignant ovarian neoplasia (AUROCC of ≥0.90). The three panels correctly classified 93 % of blinded samples comprising of various ovarian tumors and normal patient samples. In a follow-up study, 5 of the original 14 peaks were identified as transthyretin (TT, 2 peaks with m/z 13.9 kDa and 12.9 kDa), β-hemoglobin (Hb, m/z 15.9 kDa), apolipoprotein A1 (Apo-A1, m/z 28 kDa), and transferrin (TF, m/z 79 kDa) [34]. These biomarkers improved early-stage OvCa detection with AUROCC of 0.933 (compared to 0.833 for CA125). However, CA125 incorporation increased the performance to 0.959. For mucinous type OvCa, the five biomarkers were more discriminatory than CA125 alone (AUROCC of 0.959 vs. 0.613), and CA125 incorporation failed to improve detection of mucinous type OvCa. Nosov et al. extended this study to include the detection of serous and endometrioid type OvCa by analysis of Apo-A1, TT, TF, and CA125 levels [35]. This study, which included 358 serum samples from patients with benign adnexal masses, early and late stage OvCa, as well as healthy controls achieved a sensitivity of 96 % for OvCa detection (a sensitivity of 98 % for detection of early-stage endometrioid type cancer). A multicenter case–control study to validate serum biomarkers focused on 3 proteins (Apo-A1, TT, and cleavage fragment of inter-alpha trypsin inhibitor heavy chain 4) for which immunoassays were available [36]. Independent cross-validation for detection of early-stage OvCa was undertaken. These biomarkers improved detection of OvCa by CA125. The 4-biomarker combination had a sensitivity of 74 % at a fixed specificity of 97 %. Clarke et al. examined a panel of seven markers (apolipoprotein A1, truncated transthyretin, transferrin, hepcidin, b-2-microglobulin, connective tissue activating protein III, and inter-alpha-trypsin inhibitor heavy-chain 4) for OvCa detection [37]. However, in a training set, only three of these (Apo-A1, TT, and CTAPIII) performed the best with a sensitivity of 54 % at a specificity of 98 %, while CA125 in this series had a sensitivity of 68 %, and the combination increased the sensitivity to 88 %. In a validation assay, 84 % sensitivity was achieved at the set specificity of 98 %. This seven panel biomarker, however, failed to improve sensitivity beyond CA125 alone for preclinical detection of OvCa [38].
Haptoglobin 1 (HAP1) is another potential serum biomarker for OvCa. The work by Ye et al. using SELDI TOF MS and LC MS/MS identified a peak differentially expressed at a significant level between cancer and controls as alpha chain of haptoglobin [39]. This alone performed at a sensitivity of 64 % and specificity of 90 %, and together with CA125, the panel had a high sensitivity of 91 % and specificity of 95 % for detection of OvCa. Six peaks significantly upregulated in all groups of OvCa patients were identified as isoforms of haptoglobin 1 precursor [40]. This group subsequently identified a downregulated protein, transferrin, as well as the previously upregulated HAP1 in grade 3 OvCas [41]. The changes were also detected in peritoneal fluids from patients. Haptoglobin levels decreased (transferrin remained unchanged) following six cycles of taxol/carboplatin chemotherapy.
Several other potential serum proteins are uncovered for OvCa. Havrilesky et al. evaluated a panel of 9 biomarkers (HE4, glycodelin, MMP7, SLPI, Plau-R, MUC1, Inhibin A, PAI-1, and CA125) for early OvCa detection and 4 biomarkers (HE4, Glycodelin, MMP7, and CA125) for recurrence monitoring of OvCa [42]. For OvCa detection, the highest sensitivity and specificity achieved with various biomarker combinations were 80.5 % and 96.5 % for early-stage disease and 89.2 % and 97.2 % for late-stage disease. Recurrence was predicted in 100 % of the women with recurrent disease (compared to 96 % for CA125 alone). At least one biomarker was elevated earlier (6–69 weeks) than CA125, and before clinical detection of recurrence in 52 % of the patients. Of 6 proteins identified in another study, 2 (CCL18 and CXCL1) were selected by multivariate predictive model and validated [43]. Immunoassays were developed and used to screen 535 serum specimens comprising of people with OvCa, benign pelvic masses, other non-gynecologic cancers, and healthy controls. As a panel, the two biomarkers had a sensitivity of 92 % and a specificity of 97 % for OvCa detection. In combination with CA125, the sensitivity improved to 99% with healthy women, and 94 % for women with benign pelvic masses as controls, at a specificity of 92 %. Sera collected several months before cancer diagnosis from 295 women and 585 controls were profiled by MALDI MS for early detection biomarkers [44]. Two peaks, CTAPIII and platelet factor 4 (PF4), in combination with CA125 were discriminatory between cancer and controls months before cancer diagnosis, and this was earlier than predictions by CA125 alone.
Other multi-analyte serum biomarkers have proven clinically useful for OvCa. Zhang et al. tested the utility of 4-biomarker panel consisting of CA125II, CA72-4, CA15-3, and M-CSF in an artificial neural network-derived composite index for detection of early-stage OvCa [45]. The panel performed much better than CA125II alone in the detection of early-stage OvCa with a sensitivity of 71 % at a set specificity of 98 %. Hogdall et al. similarly examined 7-marker panel for utility in triaging women with pelvic masses for subsequent evaluation [46]. In multivariate logistic regression analysis, CA125, age, and the 7-proteomic biomarkers had independent predictive abilities of OvCa. The combination of these metrics (the 7-marker panel, CA125, and age) constituted the Danish Index (DK-Index). For the detection of EOC, the DK-Index had a sensitivity of 95 % and a specificity of 81 %, which was much superior to CA125 alone. Phase II multi-analyte biomarker (CA125, CRP, serum amyloid A, IL-6, and IL-8) trial for OvCa diagnosis included sera from 150 cases and 212 controls, and was validated in an independent cohort of 183 women. The 5-panel biomarker performance was significantly greater than CA125 alone in the validation cohort, as well as for stage I/II disease. Sensitivity and specificity were 94.1 % and 91.3 % for the validation cohort, and 92.3 % and 91.3 % for early-stage disease [47]. In a subsequent large-scale study, the 5 serum biomarkers were tested on sera from 222 women with EOC, 223 with benign disease, 53 with borderline OvCa, and 244 healthy controls [48]. The biomarkers were significantly elevated in sera from OvCa women compared to all the control groups. Assay performance was significantly much better than CA125 for discriminating borderline EOC from benign and healthy controls (AUROCC was 0.884 vs. 0.843 for CA125). At a specificity of 95%, the in vitro diagnostic multivariate index assay (IVDMIA) achieved a sensitivity of 69.5 % (compared to 62.5 % for CA125 alone).
13.4.6 Serum Protein Biomarkers in Clinical Use for OvCa Management
Numerous serum protein biomarkers have shown promise at the research level. Currently, however, only CA125 and HE4 are FDA approved for monitoring OvCa progression.
13.4.6.1 Serum CA125 as OvCa Biomarker
Bast and colleagues identified CA125 in 1981, when developing the monoclonal antibody OC125 against the serous OvCa-derived cell line, OVCA 433 [49]. It is a high molecular weight glycoprotein encoded by MUC16. The protein comprises of a tandem repeat of 156 amino acids at its N-terminal, a phosphorylation site at the C-terminal, and a possible transmembrane region.
The commercial immunoassay originally marketed in 1983 used the OC125 antibody. A second iteration of the test included another antibody, M11, with distinct epitope from OC125. The FDA recommends CA125 for limited intended use in the management of OvCa. However, studies show it has value in screening, differential diagnosis of a pelvic mass, treatment response and recurrence monitoring, as well as prognostic prediction.
While useful, CA125 alone faces numerous confounding factors. CA125 levels are influenced by factors such as age and race. Aging is associated with decreasing levels, and race-associated variations are observed in women after menopause. A recorded 20–50 % variation is recorded, with African-American women having lower concentrations than white women. Moreover, menstrual cycle affects serum levels, and levels increase in 1–2 % of healthy people, in 5 % of women with other gynecological conditions, and in up to 28 % of non-gynecological malignancies.
The CA125 assay is affected by analytical variables such as sample handling. Samples for CA125 analysis are therefore recommended to be assayed as soon as serum is separated; otherwise, they can be refrigerated at 4 °C for up to 5 days or frozen at −20 °C for short term (2 weeks to 3 months) or deep frozen at −70 °C for long term storage.
Serum CA125 as OvCa Screening Biomarker
Eighty percent of women with EOC have elevated serum CA125, with the following stage distribution: 50–60 % of stage I, 90 % of stage II, and over 90 % of stage III/IV women. But CA125 has not achieved the desired accuracy or evidence of reduced mortality to be a stand-alone screening test in asymptomatic women. However, women with histories of hereditary OvCa, who have a 40% risk of developing this disease over their lifetime, are recommended by the NIH consensus Development Panel to have annual CA125 screening coupled with physical pelvic examination and transvaginal ultrasound for early cancer detection. For effective screening, an OvCa biomarker must have a very high sensitivity of > 75 % and a specificity of 99.7 % to attain the acceptable PPV of 10 % among women over 50 years who have a disease prevalence of 40/100,000. Because CA125 alone, and for that matter many biomarkers, cannot reach such high accuracy, several strategies have been adopted by many investigators to attain this performance. These strategies include longitudinal measurement of CA125 (ROCA), use of CA125 with other biomarkers in panels or IVDMIAs, and the original CA125 and transvaginal ultrasound.
Serum CA125 as a Biomarker for Differential Diagnosis of a Pelvic Mass
Attempts have been made to use CA125 to estimate the risk for OvCa. Indeed, in postmenopausal women, CA125 levels above 95kU/L can discriminate women with malignant ovarian tumors from those with benign tumors at a positive predictive value of 95 %. For clinical utility, CA125 is currently used in RMI, OVA, and ROMA algorithms for such predictions (see sect. 13.4.6.3).
Serum CA125 as OvCa Treatment Monitoring Biomarker
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree