Other skin cancers
William G. Stebbins, MD  Eric A. Millican, MD
Eric A. Millican, MD  Victor A. Neel, MD, PhD
Victor A. Neel, MD, PhD
Overview
Several more common types of nonmelanoma skin cancer are presented with attention to etiology and current treatment options.
The skin is a heterogeneous organ, consisting of elements of ectodermal, endodermal, and mesodermal origin. Such a diverse group of tissues gives rise to a wide variety of benign and malignant tumors. Many of these tumors are rare and will not be discussed in this chapter. Table 1 lists the more common premalignant and malignant tumors, which we discuss in detail. These are tumors relevant to the oncologist because they have the capacity to metastasize and cause serious medical harm. We also touch on several tumor syndromes that may present with unusual benign skin tumors that, if recognized, should prompt the clinician to conduct a detailed search for an internal malignancy. Melanoma, Kaposi sarcoma, the malignant histiocytoses, and the cutaneous lymphomas are discussed elsewhere in this book.
Table 1 Common premalignant and malignant neoplasms of the skin
| Premalignant | Malignant | |
| Epidermis | Actinic keratosis Arsenical keratosis HPV-induced premalignant papules (epidermodysplasia verruciformis, bowenoid papulosis) Mucosal leukoplakia | Keratoacanthoma Basal cell carcinoma Merkel cell carcinoma Squamous cell carcinoma |
| Dermal | Dermatofibrosarcoma protuberans | |
| Malignant fibrous histiocytoma | ||
| Angiosarcoma | ||
| Appendageal | Nevus sebaceous | Sebaceous carcinoma |
| Extramammary Paget disease | ||
| Benign cutaneous tumors associated with cancer syndromes | ||
| Trichilemmomas → Cowden disease (breast/visceral tumors) | ||
| Sebaceous tumors → Muir–Torre syndrome (GI/GU tumors) | ||
| Mucosal neuromas → MEN type IIB (thyroid carcinoma/pheochromocytoma) | ||
Abbreviations: GI, gastrointestinal; GU, genitourinary; HPV, human papillomavirus; MEN, multiple endocrine neoplasia.
The incidence of nonmelanoma skin cancer (NMSC), which includes squamous cell carcinoma (SCC) and basal cell carcinoma (BCC), is increasing (Table 2). In the United States, approximately 480,000 persons were diagnosed with NMSC in 1983,1 over 1 million in 2008,2 and an estimated >3.5 million in 2014.3 The ratio of BCC to SCC among whites in the United States, Australia, and the United Kingdom is about 4:1.1, 4, 5 Together, these two tumors account for approximately 90% of all skin cancers. In recent years, the role of the sun in the causation of these common skin tumors has received much attention.6
Table 2 Nonmelanoma skin cancer statistics (U.S. 2014)
| BCC and SCC | Merkel cell carcinoma | |
| Magnitude of the problem (yearly) | >3,500,000 new patients | 1000-1500 new patients |
| Severity of the problem (yearly) | 2% deaths from SCC | 300-500 deaths |
| Disfigurement | Incidence increasing | |
| Disability |
Source: http://www.skincancer.org/skin-cancer-information/skin-cancer-facts, accessed September 2, 2014
Ultraviolet radiation in the pathogenesis of skin cancers
In the past several decades, research on the relationship between the sun and skin cancer has escalated principally because of fear of the consequences of increased ultraviolet B (UVB) radiation on the earth’s surface as a result of ozone depletion in the stratosphere. There is now general consensus over the role of sunlight in the etiology of NMSC.7, 8 Chuang and colleagues9 reported a 45-fold increase in NMSC in the Japanese population in Kauai, Hawaii, as compared with the Japanese population in Japan. Another study showed the incidence of SCC, but not BCC, in a group of Caucasian fishermen in Maryland correlated directly with the amount of sun exposure.10 A population-based study on nearly 12,000 patients also demonstrated the close correlation between chronic cumulative sun exposure and SCC.11 Geographically, the incidence of skin cancer in whites increases toward the equator, further supporting the role of sunlight in carcinogenesis.
UVB imprints a unique signature on the DNA it damages. Cellular attempts to repair this damage can lead to CC>TT mutations or C→T transitions. UVA can also induce these mutations, though likely indirectly through the creation of reactive oxygen species.12, 13 In the precursors of SCC (known as actinic keratoses), inactivating mutations of the tumor suppressor P53 harbor these UV-induced errors.14 Because P53 is involved in the transcriptional regulation of DNA repair genes, cell-cycle control genes, and the induction of cell death, damage to this important regulator by UV irradiation is one mechanism that allows for the overgrowth of damaged cells.
In addition to natural sun exposure, indoor tanning is increasingly recognized as carcinogenic. According to a meta-analysis, patients who reported ever using a tanning bed had a 67% increased risk of developing SCC and a 29% increase risk of BCC.15 Two independent case–control studies also found a greater than 60% increase in the risk of early-onset (younger than age 50) BCC.16, 17 Overall, more than 419,000 cases of skin cancer in the United States are attributable to indoor tanning annually.18 These and other similar findings led the International Agency for Research on Cancer to classify UV tanning devices as Group 1 carcinogens.19 More recently, the United States FDA reclassified tanning devices as class II (moderate-to-high risk) devices, and several states have passed legislation limiting their use among minor children. UVB and UVA (320–400 nm) also have direct and indirect effects on the cutaneous immune system, lowering cell-mediated immunity, and inducing T-suppressor cell production.20 Loss of local immunity is thought to be another factor influencing carcinogenesis.
Tumors arising from the epidermis
Actinic keratosis
Definition
Actinic keratosis, also known as solar keratosis, is a very common lesion occurring in susceptible persons as a result of prolonged and repeated solar exposures. The action of ultraviolet radiation results in damage to the keratinocytes and produces single or multiple, discrete, dry lesions with adherent scale. These premalignant lesions may, in time, progress to SCCs.
Epidemiology
Actinic keratosis affects predominantly the sun-exposed areas of fair-skinned people. The incidence in elderly whites may approach 100% in some populations.1 Actinic keratosis may appear at a much younger age (under 30 years) in individuals with an outdoor occupation, such as farmers, ranchers, and sailors, or an outdoor lifestyle. The lesions are more common in transplant recipients21 and albinos.22 It is rare in darker-skinned individuals, and almost never affects blacks, East Indians, or other Asians.
Clinical features
The onset of actinic keratosis is typically insidious and therefore often passes unnoticed for some time. The characteristic lesion is rough and gritty to palpation, similar to the feel of coarse sandpaper. Lesions are usually skin-colored or yellow brown, often with a reddish tinge, and round-to-oval, often <1 cm in diameter. They may be flat to papular, as in the hypertrophic variety of actinic keratosis (Figure 1). There may be single or multiple scattered discrete lesions, typically limited to sun-exposed areas. A pigmented variant, named spreading pigmented actinic keratosis, is a brown, slowly growing, slightly scaly lesion that tends to appear on the face and may be larger than 1.5 cm in diameter, making it difficult to distinguish from lentigo maligna.
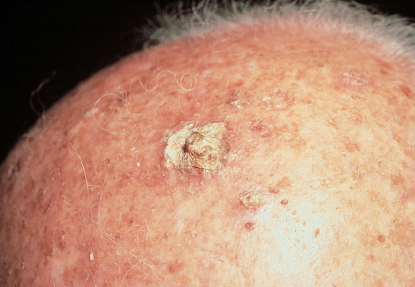
Figure 1 Actinic keratosis. Multiple discrete lesions on the scalp. These lesions are “gritty” to palpation. The largest lesion in the center of the picture represents the hypertrophic variant. This must be differentiated from a squamous cell carcinoma in situ.
Diagnosis
The diagnosis of actinic keratosis is usually based on clinical examination alone. The hypertrophic variant may sometimes be confused with early SCC. Suchniak and colleagues23 demonstrated histologically the presence of in situ or invasive SCCs in 50% of lesions diagnosed clinically as hypertrophic actinic keratoses.
Treatment
Flat actinic keratosis lesions are most easily treated with cryotherapy.24 Brief applications of liquid nitrogen with a cotton tipped swab or spray gun will suffice in the majority of cases. Retreatment may be necessary for more stubborn lesions. It is not necessary to freeze to the point of blistering. Curettage and electrodesiccation of the lesions are equally effective, but carry a slightly greater risk of scarring and dyspigmentation. The hypertrophic lesions are best evaluated by biopsy to rule out invasive SCC.
Where large areas of skin are involved, 5-fluorouracil preparations may be applied twice a day to the affected areas for up to 4 weeks. This results in a brisk reaction in the treated areas, ranging from redness, soreness, and weeping, to shallow ulceration and crusting (Figure 2), which gradually subsides after discontinuation of the cytotoxic cream. Newer formulations containing 0.5% 5-fluorouracil used once daily for 4 weeks may cause less skin irritation.25
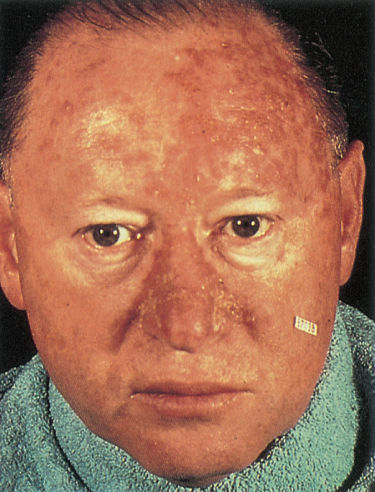
Figure 2 Actinic keratosis. Extensive actinic keratosis on the face. Note that the right half has been used as control and the left half of the forehead and the nose were treated with 5% 5-fluorouracil cream for 14 days.
Multiple additional topical approaches are available. 5% imiquimod cream has been used twice weekly for 16 weeks for nonhypertrophic, actinic keratoses of the face/scalp in immunocompetent individuals. Side effects include redness, itch and/or burning, and hypopigmentation at the local site. Imiquimod works via upregulation of inflammatory cytokines through stimulation of toll-like receptor 7 (TLR7). Topical 3% diclofenac gel has been reported to be efficacious for actinic keratoses.26 The product is used twice daily for 60–90 days. The frequency of complete response seems lower than with 5-fluorouracil or imiquimod, and skin irritation can result from treatment. The FDA recently approved another topical medication, ingenol mebutate, which works via induction of direct cell death as well as nonspecific inflammation. This medication has demonstrated comparable results to other topically applied therapies with the advantage of a being administered over a shorter 2–3 day course.27
Lastly, photodynamic therapy (PDT) employs a topically applied photosensitizer, aminolevulinic acid, which is preferentially absorbed by the premalignant cells and photoactivated upon exposure to blue or red light. PDT results in clearance of up to 90% of actinic keratoses in patients with extensive actinic keratoses of the face and scalp.28 Stinging of the treated skin occurs during light exposure. A sunburn-like reaction follows therapy.
Course and prognosis
The lesions of actinic keratosis may disappear spontaneously, but, in general, they persist if not treated. There is modest lifetime risk (<10%) of an individual actinic keratosis transforming into SCC. Marks and colleagues29 reported that 60% of SCCs arise from preexisting solar keratoses.
Keratoacanthoma
Definition
Keratoacanthoma is a common, rapidly growing low-grade tumor that may involute spontaneously, even if untreated. It is believed to originate from the hair follicles.
Epidemiology
Few studies have been done on the incidence of keratoacanthoma.30 Chuang and colleagues31 reported an incidence rate of 103.6 per 100,000, based on the small population in Kauai, Hawaii. It is most common between the ages of 60 and 65 years and is rare in persons younger than the age of 20 years. In contrast to SCC and BCC, there is no increase in frequency in old age. It is uncommon in blacks or Japanese, and is approximately two to three times more common in males. The majority of patients have a solitary lesion.
Sun exposure and exposure to chemical carcinogens such as tar are thought to be etiologic factors. The possibility of a viral etiology is still being debated. The presence of HPV has been demonstrated by DNA hybridization and polymerase chain reaction (PCR) studies.32 The possibility of a genetic defect has also been proposed because these tumors are more common in patients with the Muir–Torre syndrome than in the general population.
Clinical features
Keratoacanthomas characteristically arise on hair-bearing skin. The most common areas are the central parts of the face: cheeks, nose, ears, lips, eyelids, and forehead, as well as the dorsa of the hands, wrists, and forearms. The trunk and scalp are uncommon sites. Typically, the lesion presents as a solitary, rapidly growing, firm, dome-shaped, flesh-colored to slightly pink lesion with a plug of keratin in the central crater (Figure 3). The evolving lesion typically grows rapidly for 2–4 weeks to a size of up to 2 cm in diameter. The mature lesion involutes spontaneously after a few months, leaving a scar. The complete cycle of growth to spontaneous resolution takes 4–6 months.33 Multiple or recurrent lesions may occur, particularly in cases associated with tar exposure. Multiple lesions associated with defects in cell-mediated immunity and with multiple internal malignant neoplasms and sebaceous adenomas, as part of Muir–Torre syndrome, have been noted. There is no evidence that the solitary type is associated with internal malignancy.
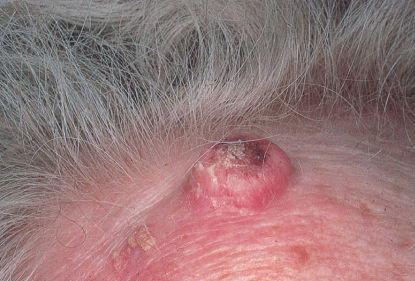
Figure 3 Keratoacanthoma. A pink, dome-shaped lesion with a central core of keratin. This rapidly growing lesion is located on the mid-forehead. Source: Courtesy of P.L. McCarthy, MD.
Diagnosis
The main differential diagnosis is to distinguish keratoacanthoma from SCC. The rapid evolution and spontaneous involution, the characteristic dome shape with a central plug of keratin, and the relatively young age of the patient are all clues to the diagnosis. In most cases, a wedge biopsy is essential to exclude an invasive SCC.
Treatment
Keratoacanthomas may resolve spontaneously. Surgical excision of the lesion will produce better cosmetic results and provide tissue for histopathologic diagnosis. Radiotherapy34 has been used successfully in lesions that cannot be distinguished from SCCs, as well as the so-called giant aggressive keratoacanthomas.35, 36 The dosage used, 4000–6000 cGy, is the same as the tumoricidal dosage used for SCCs. A biopsy of the lesion to rule out SCC prior to treatment may be prudent.
Course and prognosis
Keratoacanthoma is a low-grade tumor that carries a very good prognosis. Reports of malignant transformation or metastasizing lesions are probably misdiagnosed SCCs.
Squamous cell carcinoma
Definition
SCC is a malignant tumor arising from epidermal or appendageal keratinocytes or from squamous mucosal epithelium. There is often a history of damage by exogenous agents acting as carcinogens, such as sunlight, ionizing radiation, local irritants, or arsenic. The tumor cells have a tendency toward keratin formation. Variants include Bowen disease, an in situ SCC; verrucous carcinoma, a low grade SCC with a clinicopathologically distinct warty appearance; Buschke–Loewenstein tumor, the subset of verrucous carcinoma found on the genitals; oral florid papillomatosis, which involves the oral cavity; and epithelioma cuniculatum which involves the plantar surface.
Epidemiology
The incidence of cutaneous SCC varies greatly for different parts of the world, different races, and different life habits and occupations. The incidence was reported as 41.4 cases per 100,000 in 1983,1 and is increasing.2 Over the past three decades, SCC incidence has increased over 200% in the United states, and >700,000 cases are diagnosed annually.37
As previously mentioned, mutations in the P53 tumor-suppressor gene have been found in a number of SCCs.38 Phototherapy patients who received broadband UVB or psoralen plus ultraviolet A (PUVA) for the treatment of skin diseases, such as psoriasis or mycosis fungoides (cutaneous T-cell lymphoma), are also at increased risk.39, 40 The incidence of SCC is much higher in immunosuppressed patients, and these patients should be followed very carefully. Many dermatology departments now have dedicated clinics for immunosuppressed and transplant patients.41 Depending on the dose of immunosuppressive drugs and previous sun exposure, transplant patients have up to a 65-fold increased risk of developing SCC.42 The SCC to BCC ratio also reverses from 0.25 to 1 in the general population to 1.5-3 to 1 in transplant patients. Tumors in such patients tend to behave more aggressively.39, 43
Verrucous carcinoma of the skin is a rare tumor, with more than 100 reported cases.44 Approximately 80–90% of patients are male, with a mean age of 52–60 years. The Buschke–Loewenstein tumor is a verrucous carcinoma of the anogenital mucosa. Penile Buschke–Loewenstein tumor is the most common, with an incidence between 5% and 24% of all penile cancers. Vaginal, cervical, perianal, and perirectal Buschke–Loewenstein tumors are less common than penile ones. The etiology of these tumors is linked to HPV, particularly to HPV serotypes 6 and 11.44
The incidence of Bowen disease has not been extensively investigated. The incidence has been reported as 14 per 100,000 population in Rochester, Minnesota,45 and 142 per 100,000 population in Kauai, Hawaii.46
Progress has been made in the identification of heritable risk factors in NMSC.47 Polymorphisms of the melanocortin-1 receptor, which are linked to an increased risk of melanoma, segregate with patients at an elevated risk for both SCC and BCC.
Clinical features
SCC often arises in skin that is damaged or has been subjected to chronic irritation. Thus, the skin adjacent to the carcinoma may show evidence of solar damage, such as actinic keratoses, wrinkling, dryness, telangiectasias, and irregular pigmentation. Alternatively, there may be features of radiodermatitis from previous radiation therapy,48 a sinus tract associated with an underlying osteomyelitis, or scarring from a burn (“Marjolin ulcer”).49 Chronic venous ulcers of the lower extremities are also associated with increased risks of developing SCC.50 Chronic ulcers that show features of proliferation beyond the expected granulation process should raise the suspicion of malignant transformation.
SCC usually evolves faster than BCC, but not as rapidly as keratoacanthoma. The earliest lesion, the intraepidermal SCC or carcinoma in situ, typically appears as a scaly, erythematous plaque on sun-exposed areas, often with a sharply demarcated but irregular outline (Figure 4). Bowen disease is clinically identical to SCC in situ. Bowen disease on the glans penis is also known as erythroplasia of Queyrat.
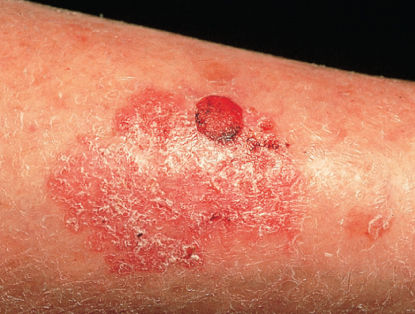
Figure 4 Squamous cell carcinoma (SCC) arising in SCC in situ. A nodule of invasive SCC on the lower leg arising within the well-demarcated, erythematous scaly plaque of SCC in situ.
Invasive SCC (Figure 5) almost always arises from a preexisting premalignant lesion or an in situ carcinoma, although de novo SCC has been reported.51 The lesion is typically an erythematous, indurated papule, plaque, or nodule, which may be polygonal, oval, round, or verrucous in shape (Figures 6 and 7). The tumor tends to increase both in elevation and diameter with time. A hallmark of SCC is its firmness on palpation. The late lesion is often eroded, crusted, and ulcerated with an indurated margin. The ulcer is often covered with a purulent exudate and bleeds easily (Figure 7). Early ulceration is often a marker for anaplastic lesions. Regional lymphadenopathy may be present either as a response to infection of the ulcer or from metastases. The latter tend to be rubbery and more irregular, and may be fixed to adjacent tissues.
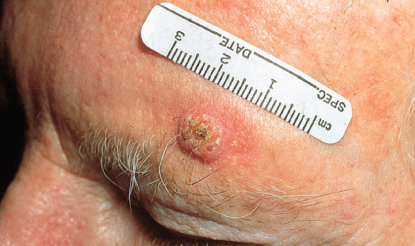
Figure 5 Invasive squamous cell carcinoma. Erythematous, hyperkeratotic nodule on the forehead resembling a keratoacanthoma (see Fig. 112–3). An incisional or excisional biopsy is necessary to distinguish the two lesions.
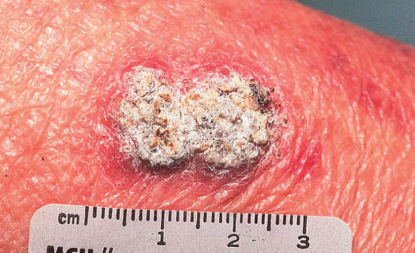
Figure 6 Invasive squamous cell carcinoma. A hyperkeratotic, crusty plaque on the forearm. Note the evidence of sun damage in the surrounding skin: wrinkling, bruising, and a lackluster appearance.
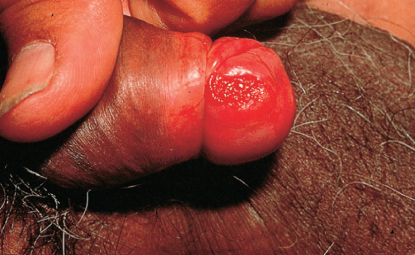
Figure 7 Invasive squamous cell carcinoma. An ulcerated lesion on the glans penis with an indurated margin. The ulcer typically bleeds easily.
Verrucous carcinoma of the skin is most commonly found on the soles of men.44 Typically, it presents as a slowly enlarging cauliflower-like mass. It is locally aggressive and may grow to a significant size. The ball of the foot is involved in more than 50% of cases. Other locations include the face, buttocks, oral cavity, trunk, and extremities. The bulk of the tumor is soft and may be foul-smelling. If left untreated, the tumor will eventually penetrate the underlying soft tissue and bone. However, metastasis is rare.
The Buschke–Loewenstein tumor most commonly affects the penile glans and prepuce of uncircumcised males, presenting as a cauliflower-like, fungating, foul-smelling tumor on the coronal sulcus. In women, it may be present on the vagina, cervix, or vulva. The Buschke–Loewenstein tumor has a tendency to infiltrate deeply, causing destruction of underlying tissues.
SCC of the lip may arise from an area of leukoplakia or actinic cheilitis, and almost always occurs on the lower lip. This tumor is typically much more aggressive than those on glabrous skin and has a higher rate of metastasis.
Carcinomas arising in longstanding radiation dermatitis tend to be histologically anaplastic and extremely aggressive, with a high rate of metastasis.52
Treatment
The choice of treatment modality depends on the degree of differentiation of the tumor and the presence or absence of metastasis. The size, shape, location of the tumor, and the predisposing factors should also be considered. In the case of a localized, well-differentiated tumor with no evidence of metastasis, the goal should be complete eradication of the lesion. In the presence of lymph node metastases or unresectable tumors, palliative treatment may be considered. The variety of modalities available for consideration include electrodessication and curettage (ED&C), excisional surgery, Mohs surgery, cryosurgery, electrosurgery, and radiation therapy.34, 53–55
Electrodessication and curettage
ED&C is an option for SCC in situ, particularly on the trunk. The primary drawback is the lack of histologic confirmation of clear tumor margins, but in properly chosen patients the recurrence rate is similar to excisional surgery.56 Invasive SCC and tumors with high-risk features have a higher risk of recurrence, however, and the preferred treatment for these tumors would allow for proper margin assessment. The larger, circular scar left from the ED&C procedure is also a deterrent for some patients.
Excisional surgery
Surgical excision with primary closure or repair with skin graft or flap is the treatment of choice for relatively small lesions with distinct borders. There should be an adequate margin of clearance of 3–5 mm to minimize the risk of recurrence. Brodland and Zitelli57 reported that margins of 4 mm were required to achieve a 95% tumor clearance rate. For invasive or large tumors (>2 cm in diameter), or tumors on high-risk areas such as the scalp, ears, nose, eyelids, or lips, Mohs micrographic surgery is the preferred approach.
Prophylactic lymph node dissection is not recommended because of the relatively low rate of metastasis. Sentinel lymph node biopsy is an emerging approach for high-risk tumors, though the specific indications remain undefined. Tumors that have metastasized to regional lymph nodes are best treated with excision, lymph node dissection, and chemoradiation.
Mohs surgery
Mohs micrographic surgery is a technique wherein a single physician excises the tumor and performs a histologic examination of 100% of the surgical margin. This technique has the lowest local recurrence rate of all treatment modalities while also allowing maximum conservation of surrounding healthy tissue, allowing for an optimal cosmetic result.57 It is the treatment of choice for tumors on cosmetically or functionally sensitive areas, as well as for recurrent tumors. It is also indicated for immunocompromised patients, tumors in previously irradiated skin, or tumors with high-risk features including poor differentiation, breslow depth ≥2 mm, diameter ≥2 cm, or perineural invasion. Appropriate use criteria for Mohs surgery have been published.57, 58
Cryosurgery
Treatment of SCC by freezing with liquid nitrogen is best restricted to the carcinoma in situ on areas that are not cosmetically sensitive. This modality has the advantage of simplicity with a high cure rate when employed in the proper situation.54, 59
Radiation therapy
Radiation therapy is an option for patients who cannot tolerate other more invasive treatment modalities. The treatment schedule is determined by the treatment modality, size, depth, and location of the tumor and the particular time-dose-fractionation schedule used. A fractionated dose provides the best cure rate and the lowest risk of adverse events, but fractionation schedules vary from 5 to 30 fractions.34 Most radiation for skin cancers in the United States is delivered using electron beam or superficial X-ray therapies with a dose between 4000 and 6000 cGy. The reported 5-year control rates for these approaches are 89% and 68% for primary and recurrent SCC, respectively.60 While reasonable, these are significantly below the 5-year cure rates for excisions or Mohs micrographic surgery. More recently, high-dose rate electronic brachytherapy has become a popular treatment approach, but 5-year control data is lacking.61 Postoperative radiation therapy can also be considered as an adjuvant treatment for certain high-risk tumors including those with perineural invasion or other high risk features (see AJCC high-risk features in the following).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree