Oral complications of cancer and their treatment
Stephen T. Sonis, DMD, DMSc  Anna Yuan, DMD
Anna Yuan, DMD
Overview
The mouth is a frequent site of direct and indirect adverse side effects of cancer therapy. In myelosuppressed patients, its diverse microbiota makes the oral cavity both a site of local infection and a potential source of bacteremias and sepsis. While about half of all patients being treated with chemotherapy or radiation therapy will develop some form of oral toxicity or infection, almost every patient receiving an aggressive myeloablative regimen or local radiotherapy to the head and neck manifests acute and chronic toxicities. The diversity of the tissues found in and around the mouth—keratinized and nonkeratinized mucosa, bone, salivary glands, and teeth—contributes to the range of susceptibility, acuity, and clinical implications of regimen-related oral complications. In almost all instances, precancer treatment elimination or control of existing oral diseases and aggressive management of oral health during cancer therapy favorably impacts outcomes.
The mouth is a frequent site of acute and chronic adverse side effects of cancer therapy. These range broadly in their nature, incidence, severity, and course, but all adversely affect patients’ quality of life, ability to tolerate therapy, overall cost of treatment, risk of local and systemic infection, and rehabilitation. Oral complications of cancer therapy are perceived as common in some cohorts such as patients with head and neck cancer (HNC), but relatively rare in others. This perception has been largely fueled by under-reported patients who worry that mentioning toxicity symptoms during treatment might, as a consequence of dose de-escalation, result in a compromise of their optimum anticancer therapy. While this phenomenon is not unique to oral complications, data surrounding the underestimates of oral toxicities are substantial. Additionally, oral side effects have been reported with many forms of developing therapies including cetuximab, antiresorptive medications, and mTOR inhibitors. It has also become clear that oral complications rarely occur in isolation. Rather, probably because of common biologic underpinnings, they predictably occur with other regimen-related toxicities.1
Overall, about 40% of patients being treated for cancers, not of the head and neck, develop some form of mouth-related problems, which range from xerostomia to mucositis.2 The frequency escalates to more than 75% for patients being treated for HNC, those who develop graft-versus-host disease (GVHD), and patients receiving aggressive myeloablative chemotherapy regimens. The symptomatic and functional consequences of oral complications include increases in analgesic and antibiotic use, length of hospital stays, hospitalizations for pain and fluid management, nursing resource use, diagnostic treating, and need for parenteral feeding. The impact on charges and costs is dramatic. In a study population of patients receiving treatment for HNC and nonsmall cell lung cancer, the incremental cost of oral mucositis alone was found to be $17,244.3 In the past, oral complications were largely considered to be inevitable, often were not recognized early, and were treated retrospectively rather than in a prospective or preventive manner. Significant progress has been made in the past decade to better define the biology and epidemiology of oral complications of treatment. As a result, interventions that target mechanisms have evolved, as has a better understanding of at-risk populations.
Pretreatment assessment
The risk of many of the side effects that impact the mouth can be successfully reduced by the elimination of existing sites of dental disease before anticancer treatment is initiated.4–6 A pretreatment dental visit is strongly recommended as it serves a range of purposes. First, it provides an opportunity for the identification and elimination of sources of active and chronic dental or periodontal infections or chronic irritation when the patient is best able to tolerate treatment with the least risk of undesirable post-treatment sequelae, such as infection or osteonecrosis. Second, oral manifestations of the primary cancer may be detected. Third, it provides an opportunity for patient education and discussion regarding the impact of the cancer and its treatment on short- and long-term oral health. Fourth, for the patient about to undergo surgical intervention for tumors about the mouth, pretreatment evaluation is critical to optimize the fabrication of prostheses. The construction of protective appliances before the start of radiation therapy may reduce the impact of treatment on scatter-induced injury.
Not surprisingly the frequency of dental disease or faulty prostheses or restoration reported on screening of patients with cancer reflects the incidence of these conditions in the general population. While positive findings of less-than-optimal oral health were reported in two-thirds of patients evaluated before stem cell transplantation (HSCT),7 pathology of such severity as to require intervention is only required in about a third of screened patients.7 Similarly, of patients with cancers of the head and neck who were screened before radiation therapy, between half and two-thirds required dental extractions, primarily because of diagnoses associated with periodontal disease.8, 9 Although elimination of possible oral and dental sites of infection before chemotherapy had a significant, favorable impact on morbidity relative to local infection and sepsis,4 definitive data demonstrating that preirradiation elimination of oral foci of infection favorably impacts outcomes is needed.10 Effective dental screening with appropriate treatment before the onset of cancer therapy results in significant cost savings, by reducing the incidence of infection during periods of granulocytopenia.11
Timing of assessment and dental treatment
If oral screening is performed so close to the initiation of cancer therapy as to preclude dental intervention, the value of the process is nullified. The ideal interval between the completion of dental treatment, particularly extraction, and the initiation of radiation therapy has been the subject of much debate. Nonetheless, given the rate by which wounds of the mouth heal, particularly extraction sites, it appears that a minimum of 2 weeks is acceptable and 3 weeks desirable.12 For patients about to undergo chemotherapy, sufficient time between the completion of dental treatment and the patient’s anticipated granulocyte nadir (<500 cells/mL) is required. In general, nonemergent dental treatment should not be performed in a typical ambulatory setting if the patient is significantly thrombocytopenic (<100,000 platelets/mL).13
Because of the acute onset of some hematologic malignancies and the need for immediate chemotherapy, pretreatment dental and oral screening in this high-risk population may not be possible. In these cases, oral assessment should be performed as close to the initiation of therapy as possible for two reasons: first, such an examination provides an important baseline for oral health and second, the finding and elimination of active oral infection in this markedly myeloablated group is often critical to their overall clinical course. Eradication of identified sources of odontogenic infection should not be delayed, as there is significant data to support the conclusion that dental extractions may be performed safely in this group if they are managed well, preferably in a hospital setting.10 The complication rate for extractions in patients with hematologic malignancies is reported to be 13%, with no effect on length of hospital stay or mortality. The most common complications include pain and bleeding. It is important to note that there is no evidence to suggest that an aggressive strategy of extraction of asymptomatic teeth has any benefit in the prevention of systemic infection.
Components of the pretreatment assessment include baseline data such as medical and dental histories; laboratory data—such as antibody status relative to herpes simplex type 1 virus—and a clinical assessment that should include an extraoral examination of the head and neck, intraoral soft-tissue examination, periodontal disease screening, and dental evaluation. Radiographic evaluation should include those films that are necessary to definitively diagnose periodontal disease and caries, periapical pathology, and impacted teeth. It is also important to assess the patient’s knowledge of, and motivation for dental maintenance. Teeth that demonstrate evidence of untreated periapical pathology, or advanced caries, or periodontal disease should be eliminated.
Patients with removable prostheses should be encouraged to minimize their use or leave them out during their cancer therapy as even subtle mucosal trauma accelerates the risk and onset of mucositis. Similarly, the removal of orthodontic bands before the start of chemotherapy is an essential component in preventing trauma to atrophied mucosa.14
Oral complications of radiotherapy
Oral complications of radiation therapy are primarily the result of acute and chronic local tissue injury. In addition, radiation-induced xerostomia may result in secondary effects on the teeth and periodontium. The dose rate, total dose of radiation, use of concomitant chemotherapy, the size of, and structures within, the radiation field are the major determinants of oral toxicity. As a result, patients being treated for tumors of the mouth, oropharynx, tongue, nasopharynx, and salivary glands are at highest risk. Patients with hypopharyngeal or laryngeal tumors are also often affected, although at a slightly lower rate. Brachytherapy tends to be more stomatotoxic than external beam irradiation. Although intensity-modulated radiation therapy (IMRT) (see 84) may spare some structures, its impact on oral mucosa is significant. Oral tissues that are directly affected by radiation include mucosa (epithelium and tissues in the lamina propria), salivary glands, bone, and muscle. In children, radiation that includes the jaws negatively affects craniofacial and dental development.15
Mucositis
Both radiation and chemotherapy can produce significant damage to the oral mucosa as a side effect of treatment. The term mucositis (ICD9 code 528.1) is preferred over stomatitis when describing mucosal injury caused by antineoplastic therapy as the latter is a generic term and can be associated with a range of infectious or traumatic etiologies unrelated to chemo- or radiotherapy. The severity and kinetics of radiation-induced oral mucositis are related to dose rate and total dose that target the oral mucosa. Local mucosal irritation, secondary infection, and xerostomia are factors that amplify the damaging effects of radiation to the tissue.
Three themes have characterized the mucositis discussion in the past 5 years: first, the pathobiology has been more fully defined; second, the commonality in mechanisms by which mucosal injury occurs has been applied to all parts of the alimentary canal; and third, mucositis rarely occurs as an isolated toxicity.16 In addition, the impact of genomics on toxicity risk, including mucositis, has become clear and is being more fully defined.
Historically, mucositis was viewed as the result of direct radiation or chemotherapy-mediated injury to stem cells in the basal layer of the oral mucosa. It was proposed that these rapidly dividing cells were indiscriminately damaged, resulting in atrophy and subsequent ulceration. Simultaneously, connective tissue injury was thought to lead to an increase in vascular permeability and tissue edema. However, studies defining the mechanisms by which mucositis occurs reveal a process that is biologically more complex. Although epithelial stem cells are the ultimate mediators of mucosal injury, it is now clear that their demise occurs by indirect, as well as direct, mechanisms.17, 18 In fact, direct clonogenic cell death of these cells is insufficient to produce the extent of clinical injury that is typically observed. Rather, a sequence of events18 triggered by the generation of reactive species in cells of the lamina propria produce a cascade of events in the endothelium, connective tissue, extracellular matrix, and the inflammatory infiltrate. This sequence begins almost immediately, following the initial exposure of the mucosa to radiation, and results in a range of molecular mediators and signals that permeate to the epithelium and cause injury, apoptosis, and necrosis.
Radiation-induced oral mucositis typically begins within the first 2 weeks of therapy, at cumulative doses of 10–20 Gy. Although clinical changes are observed at these doses, the cellular and tissue events producing these changes begin almost immediately following initial dosing (see below). Mucosal erythema, mild epithelial sloughing, and the formation of islands of hyperkeratosis characterize early changes of mucositis. These changes are accompanied by relatively mild symptoms characterized by a painful burning sensation that is analogous to a food burn such as that caused by hot cheese. Patients often have difficulty tolerating spicy foods. With the exception of the dorsal surface of the tongue, hard palate, and the gingiva, any mucosal surface of the mouth is susceptible. Most commonly affected areas are the buccal mucosa (cheeks), ventral and lateral surfaces of the tongue, and the floor of the mouth (Figure 1). The soft palate and oropharynx are also frequently involved and are consistent drivers of symptoms associated with pain on swallowing. Consequently, patients may complain of a sore throat early in their treatment.
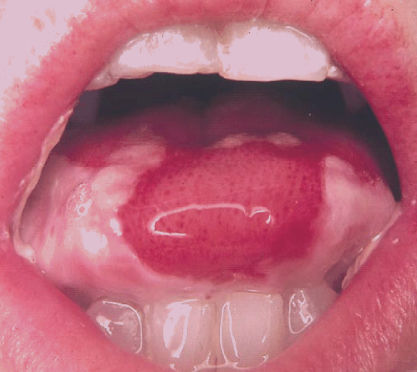
Figure 1 Severe oral mucositis with ulceration and pseudomembrane formation of the lateral and ventral surfaces of the tongue and buccal mucosa induced by myeloablative chemotherapy for conditioning before HSCT.
At cumulative doses of about 30 Gy, the integrity of the mucosa breaks down and ulceration occurs. Ulcers typically begin as isolated lesions, but then coalesce forming large, contiguous breaks in the mucosa, often covered by a collection of dead cells and bacteria in a pseudomembrane. In severe cases, the lesions may bleed (Figure 2). Ulcerative mucositis is extremely painful. Not only do ulcers cover large mucosal surface areas, but they are also deep. Patients who have undergone radiation therapy and have developed mucositis describe this complication as the most significant of their treatment.19 In many cases, mucositis results in breaks in radiation treatment, hospitalization for fluid support or pain management, and the need for parenteral feeding.20 The incremental economic cost of oral mucositis in this population is significant.3 It is important that patients about to begin treatment have some concept of the severity of mucosal injury that they are likely to develop. The typical pretreatment characterization of mucositis as “mouth sores” seems to trivialize their significance to patients. It seems likely that a more realistic description and management plan would be advantageous. In most patients, ulcerative mucositis is self-limiting and resolves spontaneously 4–6 weeks following the completion of radiation.
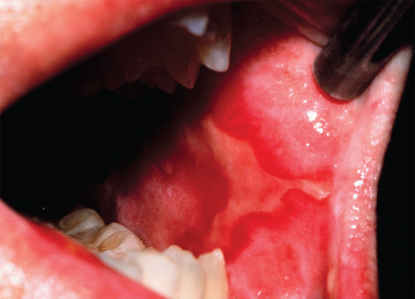
Figure 2 Severe oral mucositis with ulceration, erythema, and pseudomembrane formation of the left buccal mucosa induced by radiation therapy for treatment of an oral carcinoma.
Evaluation of mucositis
Comparisons of the stomatotoxicity of treatment regimens and efficacy assessments of mucositis interventions have been hindered by the lack of a universally acceptable scoring system for the condition. Currently, the grading systems most commonly used to describe oral mucosal toxicity are the World Health Organization (WHO) and National Cancer Institute’s common terminology criteria for adverse events (NCI-CTCAE) scales. The WHO scale combines objective findings of erythema and ulceration with the patients’ ability to eat solids, liquids, or nothing by mouth (Table 1). In its latest iteration (v.4) the CTCAE scale eliminates objective assessment of mucosal health and relies completely on symptomatic and functional (oral intake) endpoints. While this approach minimizes the clinician’s effort to assess mucositis, the dependence on patient-reported symptoms and function is complicated by analgesic use, individual pain perception, and nonmucositis-related function modifiers such as edentulism, nausea, and so on.
Table 1 Staging and management of medication-related osteonecrosis of the jaw
| Stage | Clinical presentation | Management |
| At risk | No exposed bone | Patient education |
| 0 | No clinical evidence of necrotic bone, but nonspecific clinical findings, radiographic changes, and symptoms | Systemic management, including the use of pain medication and antibiotics |
| 1 | Asymptomatic exposed and necrotic bone, or fistulae that probes to bone with no evidence of infection | Patient education; antibacterial rinses; careful follow-up |
| 2 | Exposed and necrotic bone, or fistulae that probes to bone, associated with infection as evidenced by pain and erythema in the region of the exposed bone with or without purulent drainage | Patient education; antibacterial rinses; antibiotics; pain control; superficial debridement of bone to dislodge loose fragments and smooth rough contours; careful follow-up |
| 3 | Exposed bone with pain and usually with associated soft-tissue inflammation or infection; may see osteolysis extending to the inferior border of mandible or pathologic fracture; may see extraoral fistula | Patient education; antibacterial rinses; antibiotics; pain control; palliative surgery; careful follow-up |
Source: Ruggiero et al. 2014.21 Reproduced with permission from Elsevier.
Prevention and treatment
There is currently no approved, active preventive or treatment intervention for radiation-induced mucositis in the United States. There is consensus that improved oral status may reduce the risk or severity of mucositis. Maintaining a high level of oral hygiene during treatment is thought to be beneficial.
As mucosal injury is related to the extent of mucosa exposed to radiation, the use of midline radiation blocks22 and three-dimensional radiation treatment23 may reduce the extent of stomatotoxicity.
Benzydamine hydrochloride is a nonsteroidal rinse with anti-inflammatory, analgesic, and anesthetic properties that is approved for use in the prevention and treatment of radiation-induced mucositis in Canada, Australia, and Europe. Results of a number of studies suggest its efficacy in this application.24, 25 The MASCC panel recommended the use of benzydamine among patients receiving moderate dose radiotherapy.26 There are no data to support its use in patients receiving concomitant chemotherapy.
A number of palliative barrier agents have been suggested to alleviate symptoms associated with oral mucositis. Gelclair, which has FDA approval as a device, purportedly forms a barrier on injured mucosa.27 Sucralfate, an agent that has wide use in the treatment of gastric ulcers, forms a protein-drug complex on the site of ulcerated mucosa. Its use as a rinse in the treatment of mucositis has been reported in a number of studies, although its efficacy seems inconsistent.28, 29 It is specifically not recommended in the MASCC guidelines. MuGard, a hydrogel, demonstrated significant palliation in a multi-institutional, randomized, placebo-controlled trial.30
A variety of topical agents exist for mucositis pain management. These include viscous lidocaine, benzocaine in Orabase, and suspensions of Benadryl in Kaopectate or milk of magnesia. Caphosol, a rinse originally developed as a tooth-remineralizing solution for patients with xerostomia, is an electrolyte solution of sodium phosphate, calcium chloride, sodium chloride, and purified water, which purportedly lubricates the mucosa and thereby attenuates mucositis. The solution is approved as a device, but the results of clinical trials are inconsistent.31–33 Oral aloe vera has been available for some time as a palliative agent. However, it failed to demonstrate efficacy in a phase 2, double-blind, randomized, placebo-controlled study.34 Topical palliative rinses are typically effective only for mild forms of the condition. Systemic pain management following the WHO pain ladder is often necessary. Additionally, cold foods, such as ice cream or Popsicles, may be soothing. Patients should be instructed to remove dental prostheses.
The role of microbes on the severity and course of radiation-induced mucositis is unclear.35 The strategy of mucosal decontamination as a mucositis intervention has produced conflicting results. Chlorhexidine gluconate rinses do not appear to have a role in mucositis prevention or treatment in radiation mucositis and, in fact, might exacerbate the condition.36 Lozenges containing polymyxin E, tobramycin, and amphotericin have been studied and seem to of marginal value and are not recommended.37
Given its importance as an unmet need, the development pipeline for mucositis is rich with agents that target key elements in its pathogenesis. Clinical trials are currently in progress in which drugs targeting oxidative stress, the innate immune response, and proinflammatory elements are being studied.38 Low-level laser therapy is also being investigated for its potential utility as an intervention of oral mucositis. While results of clinical trials are encouraging, the lack of substantive studies defining its biological effects as related to tumor response is troubling. More investigation is clearly needed to assure that its impact on premalignant and malignant tissue is benign.39
Xerostomia
Xerostomia is one of the most consistent and bothersome side effects of radiation therapy in which the salivary glands are included in the field of treatment,40 and may be exacerbated by concomitant chemotherapy. The effects of radiation on salivary flow are variable, and symptoms of dry mouth may not correspond to observed salivary flow. Xerostomia is caused by the effects of radiation on acinar cells, especially of the serous glands (parotid). Consequently, inflammation, degeneration, and fibrosis of the glandular parenchyma occur. The extent, duration, and degree of recovery are functions of the dose rate, total dose, and radiation port. Onset of xerostomia may be noted as early as 1 week following the start of radiation (cumulative dose of 10 Gy).41 The saliva turns thick and ropey as serous function is diminished, but mucous production remains. Patients whose radiation to the ear and neck is in cumulative doses of 60 Gy more often develop irreversible xerostomia, with an 80% loss in salivary gland function.42 Spontaneous recovery is unlikely for patients with xerostomia persisting for 12 months or longer.43 With lesser doses of radiation, however, inflammation and edema of glandular tissue often spontaneously disappear within a year of the completion of treatment.44
In addition to functional changes caused by xerostomia, such as dysphasia and alteration in taste, loss of saliva is also associated with a reduction in oral clearance, diminished salivary immunoglobulin A (IgA) levels, and salivary antibacterial enzymes. Consequently, patients with xerostomia are susceptible to increases in local oral infections including caries, periodontal disease, and candidiasis. Aggressive oral hygiene to reduce the tooth-borne bacterial load is critical to reducing the risk of dental disease.
Radiation-induced caries can be a common problem in patients with xerostomia.42 Changes in salivary composition, decreases in buffering capacity, and loss of the cleansing action of saliva result in the accumulation of bacteria, increases in local cariogenic flora, and tooth decalcification with consequent caries development.45, 46 Typically, radiation caries present with lesions at the cervical margins of teeth, which then rapidly progress. Decalcification (white, chalky enamel) of the incisal edges of the teeth may also be noted. In addition to tooth loss, a major consequence of uncontrolled caries may be abscess formation in patients who are at risk for osteoradionecrosis (ORN).
Four goals should be considered for the prevention and treatment of xerostomia. Preservation of salivary function is critical. Whenever possible, tissue-sparing techniques aimed at minimizing the amount of salivary tissues exposed to direct radiation should be used. While bilateral field radiation may result in an 80% reduction of salivary flow, mantle irradiation typically causes only a 30–40% decrease. Parotid sparing using three-dimensional treatment or intensity-modulated radiotherapy techniques offers the greatest chance of glandular repair.47 Stimulation of salivary flow should start simultaneously with radiation therapy (XRT), as should an anticaries regimen to protect the dentition. Replacement of reduced secretions may be introduced as soon as needed.
Stimulation of salivary flow may be accomplished through local or systemic means. Sucrose-free lemon drops or sugarless chewing gum may be used. Cinnamon- or mint-flavored mints or gum should be avoided as they may irritate the mucosa.
Drug therapy may also help to stimulate parotid flow.48 Of the cholinergic agents, pilocarpine has been best studied and found to stimulate parotid function, but not submandibular or sublingual gland function in patients with Sjögren syndrome and radiation-induced xerostomia.49, 50 Other agents such as bromhexine, anetholtrithion, bethanechol HCl, potassium iodide, neostigmine, and reserpine have been used for salivary stimulation, but data substantiating their efficacy are scant. In contrast, substantial data exist to support the use of pilocarpine HCl tablets to stimulate salivary flow in patients with radiation-induced xerostomia.51 In cases in which pilocarpine is used after patients have completed radiation treatment and are symptomatic, at least some residual salivary function must be present, and patients should be cautioned that clinically significant improvements in salivary flow may not be realized for up to 3 months following the initiation of treatment. Alternatively, pilocarpine may be prescribed to start simultaneously with radiation therapy. In either case, the typical dose of 5 mg given three times daily may be titrated depending on the patient’s response and manifestation of side effects.
Amifostine, a nonprotein, free-radical scavenger has been approved as a cytoprotective agent for salivary glands to prevent radiation-induced xerostomia.52 The recommended dose for amifostine is 200 mg/m2, administered once daily as a 3-min infusion, starting 15–30 min before standard fraction radiation therapy. The need for intravenous infusion, frequency of dosing, cost, and potential side effects have limited amifostine’s adoption. Furthermore, the results of a recent meta-analysis suggest that amifostine’s efficacy is tempered among patients receiving radiation regimens in which concomitant chemotherapy is also administered.53
Salivary replacement can be accomplished with the use of saliva substitutes or artificial saliva.54 Most of these materials contain carboxymethylcellulose and may provide transient symptomatic relief of mucosal dryness. Saliva substitutes are available as over-the-counter rinses or sprays and are most effective if used before meals and at bedtime. A number of toothpastes and chewing gums have been developed specifically for use in patients with xerostomia.
Exciting new regenerative approaches to restore salivary gland function have recently been reported, but are still limited to preclinical studies.55
The most effective protective strategy for radiation-induced caries is the aggressive use of topical fluorides.56 Topical fluoride supplements should be initiated at the start of radiation treatment. Continuation of fluoride following the completion of radiotherapy is critical, especially in patients who develop xerostomia. Fluorides for dental use come in three forms: rinses, gels applied by tooth brushing or in customized trays, and drops also used in trays molded to fit over patients’ teeth. Patients in whom xerostomia is anticipated should have fluoride trays fabricated before the initiation of radiotherapy. Fluoride gel or drops are placed in the trays and applied by the patient each day. Use of tray-borne application can be supplemented with acidulated fluoride rinses; generally, the use of rinses in the morning and trays before sleep is most effective and easiest for patients. Acidulated fluorides tend to work best, although neutral fluoride rinses are available for patients with mucositis in whom acidulated material might be irritating, or for patients with porcelain prostheses in whom pitting of the restorations might occur. The supplemental use of a remineralizing toothpaste should also be considered.57 Aggressive oral hygiene is to be encouraged, and patients should be seen by a dentist frequently. Regular dental visits are critical to insure early detection and intervention of caries and periodontal disease.
For patients who cannot tolerate trays because of gagging or mucositis, fluoride gels may be applied with a toothbrush, either as 1.1% sodium fluoride or as 0.4% stannous fluoride. The latter appears to be more efficacious. Patients should be instructed to avoid sucrose.
Loss of taste is a transient, but bothersome, sequelae of head and neck radiation.58 The severity of taste loss increases rapidly up to doses of 30 Gy, but then usually plateaus. Patients who receive doses of 30 Gy or more may lose their ability to distinguish salt or sweet tastes. Fortunately, hypogeusia is typically transient and taste begins to return within 1–2 months after the completion of treatment. Total recovery may take up to a year. If there does not seem to be progression to improvement following radiotherapy, candidiasis should be ruled out.
Osteoradionecrosis
Of all of the oral complications of head and neck radiation, one of the most significant is ORN.59 First described in 1927, ORN results in the denudation of soft tissue and exposure and necrosis of bone.60, 61 Although not limited to the jaws, it frequently occurs at this site. ORN causes a painful, chronic, open, and foul-smelling wound that is typically of great distress to the patient. Most cases ultimately heal with conservative treatment, but the course is usually prolonged. ORN was attributed to a triad of trauma (often tooth extractions), radiation, and infection.62 Subsequent studies suggest, however, that ORN represents a defect in wound healing rather than a true osteomyelitis.63 The etiology appears to relate to diminished vascularization as a consequence of XRT.64 Histologic changes of thickened arterial and arteriolar walls substantiate this hypothesis. The finding of cultivable and noncultivable bacteria may suggest an infectious component.65
No consensus exists concerning the overall frequency of ORN, although reported ranges vary between 4% and 44%. While approximately 15% appears to be the preponderant experience,59, 63 a recent meta-analysis reported that only 2% of HNC patients are at risk.66 The mandible is involved more often than the maxilla, which probably reflects the difference in blood supply and vascularity of the two bones. Time until onset of ORN following XRT is variable. Some authors have described ORN as early as 2 weeks after XRT, others report it as a late condition. Most cases occur within the first 3 years after XRT (74%). Equally controversial is the rate at which ORN risk diminishes with time after the completion of XRT, although it seems clear that the risk never reaches zero.67
A number of risk factors for ORN have been positively identified.68 Men have been reported to have a risk for ORN that is threefold higher than women.61 Patients who are edentulous are twice as likely as patients with teeth to develop ORN. Furthermore, the frequency of ORN increases dramatically in individuals with active dental disease (e.g., periodontal disease, caries, periapical disease, and poorly fitting prostheses).59 Fifty percent of cases appear to be associated with tooth extraction following radiation. These findings strongly support pre-XRT dental evaluation and aggressive repair and removal of diseased teeth. The field size, dose rate, and total dose of XRT have a marked effect on the frequency of ORN. Patients who receive cumulative doses of 65 Gy or more to the mandible or maxilla are more likely to develop ORN than are patients receiving lesser doses. Use of three-dimensional radiation techniques has resulted in a slight reduction in ORN risk.69 Patients with tumors that are adjacent or contiguous with bone are also at higher ORN risk. It is likely that this finding is due to the inclusion of bone in the radiated field as the volume of bone exposed to XRT has a direct impact on ORN risk. Poor nutrition and immune status also appear to predispose to the condition. Diagnosis of ORN is usually based on clinical findings. In cases in which the diagnosis is questionable, magnetic resonance imaging may be of value.70
Treatment of ORN is based on the severity and chronicity of the condition.71 Fortunately, most lesions (up to 60%) eventually heal in approximately 6 months with conservative therapy consisting of local debridement, saliva irrigation, and oral antibiotics.72 Results of studies in which pentoxifylline, used for its anti-TNF activity, was assessed are inconsistent.73, 74
Lesions showing no improvement or demonstrate progression require more aggressive therapy. For these cases, surgical debridement and hyperbaric oxygen (HBO) may be indicated.75, 76 In extensive cases, radical resection of involved bone with immediate microvascular reconstruction has been used successfully in patients who have failed more conservative treatment, including HBO.77
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








