Fig. 16.1
The approach to patients with stage IV rectal cancer
All Sites Resectable at Presentation: Curative Intent
In those with resectable primary and metastatic disease , the goal of therapy is curative. The resectability of liver metastases was historically defined by the characteristics of the tumor deposit(s), including size, number, and location within the liver. However, newer resectability criteria focus on the remaining liver following metastasectomy and have increased the number of patients eligible for liver resection. These criteria must all be met and are (1) R0 resections (i.e., complete resection with microscopically clear margins) of all disease sites, including the primary and the intra- and extrahepatic metastatic disease sites, (2) sparing of at least two adjacent liver segments, (3) preservation of the vascular inflow and outflow and the biliary drainage of the remaining liver segments, and (4) preservation of a minimum of 20% future liver remnant (FLR) in normal livers, 30–60% FLR for livers injured by chemotherapy, steatosis, or hepatitis and 40–70% FLR for liver cirrhosis [16, 17]. Further strategies to increase the number of patients eligible for hepatic resection include portal vein embolization [16].
For pulmonary disease , resectability criteria are less well defined and mainly include the ability to achieve an R0 resection. In the metachronous literature, some have noted that patients with three or more pulmonary metastatic deposits are twice as likely to experience a recurrence [18]. In a recent meta-analysis of retrospective single-center experiences, multiple lung metastases, thoracic lymph-node involvement, and elevated pre-thoracotomy carcinoembryonic antigen levels all increased the risk of death [13].
There are numerous strategies in existence that vary in the timing of surgery for the primary and metastases, systemic chemotherapy, and chemoradiation. Timing and sequencing of therapy remain debated and controversial. Early involvement and discussion with surgeons, in conjunction with medical oncologists and radiation oncologists in a multidisciplinary fashion, is crucial. The most recent iteration of the National Comprehensive Cancer Network (NCCN) 2014 guidelines recommend preoperative combination chemotherapy for 2–3 months, with or without subsequent chemoradiation, followed by surgery [19]. Resection of the primary and metastasis can then be undertaken simultaneously or in a staged fashion. Chemoradiation can be considered postoperatively if not given preoperatively [19].
5-Fluorouracil-based chemotherapy (with oxaliplatin or irinotecan) has been shown to improve median overall survival for patients with metastatic colorectal cancer in randomized controlled trials [20, 21]. In particular, the trial by De Gramont et al. included patients with synchronous disease (i.e., stage IV at presentation), who accounted for 66% of the study population [20]. More recently, the addition of the antiangiogenic agent bevacizumab has further improved median overall survival in metastatic colorectal cancer [22]. Because of its antiangiogenic properties, there have been concerns surrounding impaired wound healing leading to increased perioperative morbidity with the addition of bevacizumab to preoperative chemotherapy regimens. However, if stopped at least 8 weeks prior to surgery, bevacizumab does not seem to increase morbidity [23]. Additional recent randomized trials also examined the use of anti-epidermal growth factor receptor agents cetuximab and panitumumab in the setting of metastatic colorectal cancer. Ye et al. found that for colorectal liver metastases, the addition of cetuximab to oxaliplatin- or irinotecan-based chemotherapy significantly improved the overall response rate from 29% to 57% compared to chemotherapy alone [24]. The overall response rate in this study included both complete and partial response according to the response evaluation criteria in solid tumors (RECIST) guidelines [25].
If considering upfront chemotherapy followed by surgery, the severity of symptoms relating to the primary must be taken into consideration. In cases of clinical obstruction, surgical diversion with an ostomy or endoluminal stent placement may be appropriate prior to initiating chemotherapy. Unfortunately, there are also known risks of hepatotoxicity relating to preoperative chemotherapy, specifically with oxaliplatin and irinotecan [26], and prospective data to support preoperative chemotherapy in clearly resectable liver metastases is lacking [27].
Timing and Sequence of Surgery
The goal in this subgroup of stage IV patients is complete surgical resection of all disease sites. Thus, the different surgical strategies include (1) rectal resection first, followed by hepatic resection; (2) hepatic resection first, followed by rectal resection (the “liver first” approach); or (3) simultaneous rectal and hepatic resections. The first two strategies are often collectively termed “staged resections.” Rectal resection-first approach has traditionally been the path most taken. However, more recently, investigators have suggested the liver-first approach as a strategy to ensure that resectable metastases are treated without delays caused by potential complications from rectal surgery.
While there are no randomized prospective studies, several retrospective series have reported on the feasibility of the liver-first approach. The majority of these studies included both colon and rectal cancer patients [28–31]. Verhoef et al., along with Ayez et al. in a follow-up study, reported on their experience with the liver-first approach specifically in patients with locally advanced rectal cancer and synchronous liver metastases [32, 33]. These patients received a median of five cycles of oxaliplatin- or irinotecan-based chemotherapy with or without bevacizumab, then underwent hepatic resection, followed by long-course radiotherapy with or without capecitabine, and then completed the protocol with rectal resection. Of the 42 patients identified, 31 completed their protocol, with 11 remaining patients developing progressive disease precluding rectal surgery. More recently, Buchs et al. reported their experience with a similar liver-first strategy for rectal cancer [34]. Of the 34 patients included, 33 completed their protocol. With respect to the rectal cancer surgery, the rate of a positive distal margin in this study was 6.1% (seen in two patients). Neither of these patients received the long-course chemoradiotherapy prior to their rectal cancer surgery due to the acute development of obstruction and perforation. They both experienced a pelvic recurrence. There were no patients with a positive circumferential resection margin. Three patients experienced a complete rectal pathologic response. Overall, five of 33 patients experienced a pelvic recurrence. The 5 years overall survival was 52.5% in this cohort.
There have also been some retrospective reports of simultaneous colorectal and hepatic resections. A case series from the Mayo Clinic described 45 patients who received simultaneous R0 resections with a 5 years overall survival of 32% [35]. There were no significant differences in local recurrence rates between the 18 patients who received neoadjuvant (i.e., preoperative) pelvic radiotherapy and the 27 patients who did not. There were no perioperative mortalities. There were 26 postoperative complications in this group. In another retrospective series, patients who underwent simultaneous resections were compared to patients who underwent staged resections in a multi-institutional analysis [36]. There were more patients in the simultaneous group (40%) with rectal primaries than in the staged group (23%). Simultaneous resections resulted in a significantly shorter combined length of hospital stay compared to staged resections. In the subgroup of minor hepatectomies (resection of less than three Couinaud hepatic segments), the mortality and major morbidity rates were similar between the simultaneous and staged resection groups. However, simultaneous resections with major hepatectomies (resection of at least three Couinaud hepatic segments) had significantly increased mortality and major morbidity when compared to staged resections. The authors concluded that simultaneous resections with minor hepatectomies are safe but that consideration should be given to simultaneous resections with major hepatectomies. Interestingly, there was not a similar subgroup analysis comparing the impact of colonic resections to rectal resections.
On a national level, a recent retrospective study using the American College of Surgeons National Surgical Quality Improvement Program data demonstrated that patients who underwent simultaneous resection had more perioperative adverse outcomes, longer operative time, and longer length of stay than colorectal resection or hepatic resection alone [37]. However, the authors were unable to perform a direct comparison of these outcome variables in the simultaneous group to the cumulative outcome variables of the staged colorectal and hepatic resections.
Thus, true comparisons between the simultaneous and the staged resections are not readily available. Currently, the strategy employed in the surgical management of the rectal primary and liver metastasis is determined by the review of the individual patient and by the surgeon(s)’ level of comfort with simultaneous resections.
The Role of Pelvic Radiation
The role of pelvic radiation in the treatment of rectal cancer is thought to lie mainly in local control [5, 7, 8]. In those with clearly resectable stage IV rectal cancer, neoadjuvant treatment of the primary with 6 weeks of chemoradiotherapy followed by an 8-week wait before rectal resection can mean delays in the administration of systemic chemotherapy or in the surgical management of the metastatic disease. Butte et al. retrospectively examined the patterns of failure in stage IV rectal cancer patients with resectable liver metastases managed at the Memorial Sloan Kettering Cancer Center [38]. After the complete resection of all sites of disease and a median follow-up of 44 months, 70% of the entire cohort developed a recurrence. Ten percent of the cohort developed a pelvic recurrence in combination with recurrence at other sites, and 4% developed an isolated pelvic recurrence. Approximately half of the patients in this series received perioperative pelvic radiation, with the majority of these patients receiving neoadjuvant radiation. The receipt of pelvic radiation was not found to be associated with the rate of pelvic recurrence. The disease-specific survival at 5 years was 51%. It was not associated with pelvic recurrence or with the receipt of pelvic radiation.
Some authors have also examined the utility of adjuvant (i.e., postoperative) pelvic radiation in the setting of stage IV rectal cancer. In Korea, there are several retrospective series comparing the use of adjuvant chemotherapy to adjuvant pelvic chemoradiotherapy in stage IV rectal cancer [39–41]. In one of these studies, following simultaneous rectal and hepatic resections, there was no difference in median disease-free survival or in overall survival between patients who received adjuvant chemotherapy compared to patients who received adjuvant chemoradiotherapy. Approximately 42% of the adjuvant chemotherapy group received oxaliplatin- or irinotecan-based chemotherapy, with the remaining receiving fluoropyrimidine therapy alone. The groups were not equal, as there were higher proportions of patients with abdominoperineal resections and node-positive disease in the chemoradiotherapy group than in the chemotherapy group. The majority of recurrences were distant only (67%), as compared to locoregional only (3%), and distant plus locoregional recurrence (2%). A second retrospective series revealed similar findings of a lack of difference in overall survival or disease-free survival between adjuvant chemotherapy and adjuvant concurrent chemoradiotherapy [40]. These two series stand in contrast to the third retrospective series demonstrating a benefit in pelvic-failure-free survival in stage IV rectal cancer patients receiving adjuvant radiation compared to patients receiving adjuvant chemotherapy [41]. However, no overall survival benefit was seen.
When managing the benefits of local control, clinicians may be able to predict who will derive the most benefit from pelvic radiation. The MERCURY study has suggested a sensitivity of 64% and specificity of 91% for the use of pelvic MRI in predicting a positive circumferential resection margin [42]. A positive circumferential margin was predicted on MRI if tumor is seen to be within 1 mm of the mesorectal fascia on MRI. When followed prospectively, a significant difference in overall survival was observed between patients with an MRI-predicted positive circumferential resection margin compared to those with an MRI-predicted negative margin. A similarly significant difference in local recurrence rates was also observed between these two groups. A recent meta-analysis of the diagnostic accuracy of pelvic MRI for assessing T and N stages and circumferential resection margin included the MERCURY study along with 20 other studies, nine of which were retrospective [43]. The pelvic MRI was found to be most accurate for circumferential resection margin status. In addition, T stage assessment by pelvic MRI has a sensitivity and specificity of 87% and 75%, respectively. Accuracy for N stage was found to be relatively poor.
In the stage IV setting, the use of pelvic MRI can be helpful for determining the patients with locally advanced disease who will benefit the most from pelvic radiation in addition to surgery as a means for local control (i.e., those most likely to have a positive circumferential resection margin). In these patients, “short-course” radiotherapy can be used to decrease the concerns of delaying surgery and systemic chemotherapy that is seen with the traditional neoadjuvant chemoradiation regimen (6 weeks of chemoradiation followed by an 8-week wait) that is typically given in North America. Short-course radiotherapy is used more commonly in Europe and consists of 5 days of 5 Gy pelvic radiation per day, with rectal surgery taking place within a week of completion of pelvic radiation [5, 6, 9]. Alternatively, postoperative chemoradiation may be employed for local control. However, in general, pelvic radiation likely plays a diminutive role in the curative management of stage IV rectal cancer given that the majority of these patients recur distantly.
Patients with Potentially Curative Disease
Approximately 70–80% of patients with colorectal liver metastases are considered unresectable at initial presentation. However, a proportion of patients with initially unresectable disease can potentially be converted to resectable disease. In such cases, it is still possible to approach these patients with a curative intent. Select patients treated with modern chemotherapy regimens can be “downstaged” and converted to resectable disease with improvements in survival. The NCCN recommends restaging studies to reassess resectability every 2 months while on chemotherapy.
The resection rate following chemotherapy for initially unresectable colorectal liver metastases was reported to be 32.5% in a series from Italy [44]. Two-thirds of the study population had synchronous disease. A total of 40 patients received irinotecan, 5-fluorouracil, and folinic acid therapy with assessments for response every 6 cycles (12 weeks). Patients underwent resection as soon as their disease was reassessed and deemed converted to resectable. The presenting factors most favorable for resection after chemotherapy were small number of metastases, right lobe involvement only, and presence of large lesions. The median disease-free survival in the patients who were resected was 14.3 months, while the median progression-free survival in unresected patients was 5.2 months.
Another group reported treatment with oxaliplatin, 5-fluorouracil, and leucovorin resulting in a lower conversion rate of 13%: 138 colorectal cancer patients underwent hepatectomy out of an initial 1104 patients with unresectable metastatic disease after an average of ten courses of chemotherapy [45]. Assessments were performed every four courses of chemotherapy. Perioperative mortality and morbidity were 0.7% and 28%, respectively. The 5 years survival of patients with rectal primaries who were successfully downstaged to receive metastasectomy was 25%.
Therefore, in those successfully downstaged, there are reported survival benefits to subsequent surgical resection. Relatively frequent reassessments to determine treatment response are important in selecting for these patients. In order for a patient to be successfully downstaged and considered for treatment with a curative intent , all apparent sites of disease at diagnosis must be amenable to complete surgical resection.
Patients with Unresectable Disease and Symptomatic Rectal Primaries
In patients presenting with unresectable metastatic disease , the goal of therapy is palliative, and not curative, in nature. Metastatic disease that is unlikely to become resectable even with upfront chemotherapy treatment includes diffuse extrahepatic and extra-thoracic disease including peritoneal carcinomatosis, mediastinal involvement, miliary hepatic, or pulmonary involvement. In addition, patients who are not surgical candidates due to medical comorbidities also fall in this category. On the other hand, when patients present with symptoms or complications related to their primary tumor, resection may be appropriate despite the presence of unresectable metastatic disease. These complications include bleeding, obstruction, perforation, and pelvic pain. Surgical management in this situation must be tailored to the patient’s symptoms and ability to tolerate the resection, as well. Other non-resection strategies for management of the symptoms include systemic chemotherapy, chemoradiotherapy, diverting ostomy, and endoscopic stent placement. Symptoms of obstruction may be relieved with diverting ostomy, surgical resection, or endoscopic stent placement. However, pelvic pain and/or bleeding is best managed with chemoradiotherapy or surgical resection. The strategy chosen should minimize morbidity while providing maximal symptom relief, with minimal delays to systemic chemotherapy administration, if possible.
Pelvic Radiation for Palliation
There is some evidence that radiotherapy is a reasonable alternative to surgical management (resection or diversion with an ostomy) of symptomatic rectal primaries. A phase II study enrolled previously untreated stage IV rectal cancer patients with symptomatic primaries who had obstruction (53%), bleeding (27%), and pain (20%) to receive short-course radiotherapy followed by capecitabine and oxaliplatin [46]. The short-course radiotherapy consisted of five fractions of 5 Gy pelvic radiation given over 5 consecutive days. Twenty percent of patients required palliative surgery secondary to local symptom progression. Seven patients received diverting ostomies (five for obstruction and two for perianal fistulae), and one patient required local tumor excision for bleeding. On a quality of life questionnaire, 30% reported complete symptom resolution, and 35% reported significant improvement in their symptoms during the course of follow-up. The remaining 35% reported poor palliative effect.
Thus, for patients who would not otherwise tolerate a resection and who wish to avoid a diverting ostomy, pelvic radiation may be an option for palliation of pelvic symptoms, including obstruction, bleeding, and pelvic pain.
Endoluminal Stents
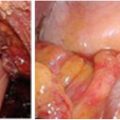
In the setting of acute malignant obstruction, the surgical options again include resection or diversion. More recently, the use of self-expanding metallic stents to palliate obstructive symptoms from rectal cancer has been reported in the literature. Stents are typically placed and deployed using a combination of endoscopy and fluoroscopic guidance. There are typically two broad indications for endoscopic stent placement: (1) palliation and (2) bridge to surgery. In the first setting, the self-expanding metallic stents are placed for definitive symptom (usually obstruction) control (see Fig. 16.2) for palliation. These stents are not removed, and should tumor ingrowth occur, a repeat endoscopic procedure is performed, if possible. In the second setting, as a bridge to surgery, stents are placed for patients who are acutely obstructed but are candidates for surgical resection. Therefore, the stent is placed in order to allow for temporary relief from the obstruction and preparation for surgery. The patient then undergoes definitive surgical resection shortly after stent placement. In this setting of stage IV rectal cancer with unresectable disease that is managed with a palliative intent, the stents are typically placed for the first indication.
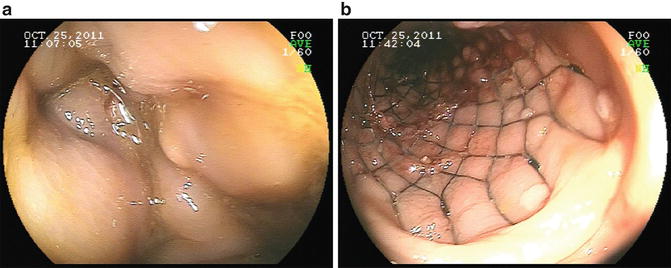

Fig. 16.2
The use of endoluminal self-expanding metallic stent for obstructing primary lesion. Before (a) an obstructing lesion was found on colonoscopy. After (b) the obstruction was relieved with the placement of a self-expanding stent. Mucosal abnormalities consistent with the primary lesion can be seen abutting the stent
A Japanese phase II study evaluated 33 patients with unresectable obstruction of the rectum or sigmoid colon treated with stenting [47]. The stent was effective in 82% of the patients. One stent was removed secondary to anal pain. The level of the tumors treated in this study was proximal to 5 cm above the anal verge. No stent migration was observed; however, the medial duration of follow-up was only 78 days.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree