Oncologic emergencies
Sai-Ching Jim Yeung, MD, PhD, FACP  Carmen P. Escalante, MD
Carmen P. Escalante, MD
Overview
Cancer and its treatment can lead to oncologic emergencies. This chapter discusses the approach to acute emergency problems in cancer patients. A list of emergent problems has been selected for focused discussion. Sudden cardiopulmonary arrest is discussed along with special consideration in resuscitation of cancer patients. Arrhythmia, superior vena caval syndrome, pericardial tamponade, and acute hemorrhage are important cardiovascular emergencies. Tumor lysis syndrome can be rapidly fatal, and early recognition and treatment are very important in preventing disastrous outcomes. Pulmonary problems include airway obstruction, pleural effusion, hemoptysis, pneumothorax, and pulmonary embolism. Neurological emergencies include spinal cord compression, brain herniation, and status epilepticus. Neutropenic fever is perhaps the most frequently discussed important topic in oncologic emergency. Other important issues such as perforated viscus, anaphylaxis, and cytokine release syndrome are also discussed. Oncologists and emergency physicians must be aware of these potentially serious acute complications of cancer patients in order to initiate appropriate treatments in a timely manner.
Introduction
An oncologic emergency is an acute condition that is caused by cancer or its treatment and that requires intervention as soon as possible to avoid mortality or severe morbidity. Cancer patients are more likely to require emergency care than noncancer patients. Physical debilitation, altered hemostasis, and impaired immunity due to malignancy or its treatment also make cancer patients vulnerable to accidents and mishaps in everyday life. Because cancer patients have unique concerns and changes in physiological status, emergency care providers need to adapt to their special needs.
The emergency care of cancer patients is evolving into a hybrid discipline—a cross between oncology and emergency medicine. There are many types of problems for which cancer patients present to an emergency care facility, and in-depth discussions would fill volumes.1–3 Because of page constraints and coverage of some relevant topics in other chapters of this book, this chapter will cover only selected topics.
Approach to acutely III cancer patients
Cancer patients often have comorbidities such as coronary heart disease, diabetes mellitus, and chronic obstructive pulmonary disease. Some of these may be attributable to the same risk factors for carcinogenesis (i.e., old age, diet, cigarette smoking, or sedentary lifestyle). Emergency care providers caring must assess the extent of the malignancy, the response to treatment, the overall prognosis, and the patient’s and family’s wishes in order to formulate an appropriate treatment plan (Figure 1). The majority of cancer patients who are approaching their ends of life do not want “heroic” measures, and addressing advance directives and do-not-resuscitate (DNR) orders in a timely manner may improve the quality of life in the weeks before death.4
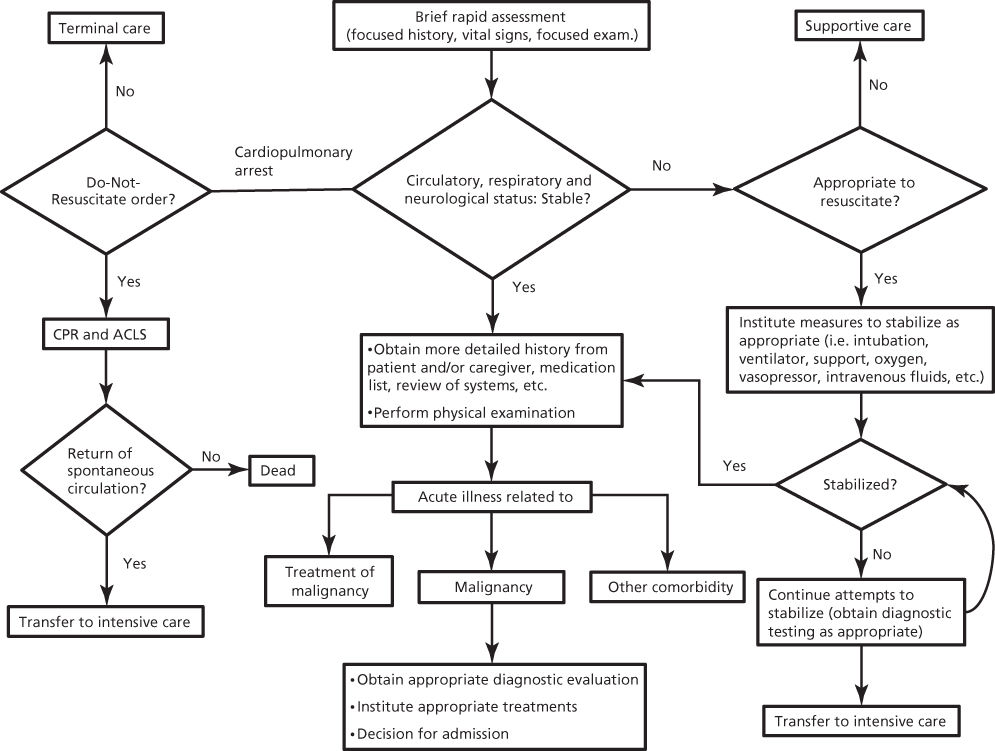
Figure 1 Approach to acutely ill cancer patients.
First, the patient should be rapidly assessed. This assessment should include the chief complaint, a focused history, vital signs, and a quick overall physical assessment. If the patient is unable to relay the history of present illness, a family member, companion, or caregiver may provide pertinent information. Intervention for unstable vital signs should be initiated immediately. In case of cardiopulmonary arrest, appropriate guidelines are followed (http://www.acls.net/aclsalg.htm).5 Once the patient is stabilized, thorough history and physical examination should be completed. For the majority of cancer patients with emergencies, a comprehensive evaluation is necessary. The emergency may be due to the cancer, cancer treatments, or comorbid conditions, all of which should be considered in the differential diagnosis.
Circulatory oncologic emergencies
Sudden cardiopulmonary arrest
Most deaths are preceded by cardiopulmonary arrest. Resuscitation is more likely to succeed when cardiopulmonary arrest was caused by an acute reversible insult rather than by a steady irreversible decline in bodily functions. The success rate of resuscitation and the hospital discharge rate of resuscitated patients are similar for cancer patients and noncancer patients.6 A meta-analysis of inpatient resuscitation (including cancer patients) estimated that the probabilities of successful resuscitation and being discharged alive are about 30% and 12%, respectively.7 Similarly, for cancer patients with out-of-hospital arrest who received resuscitation at the Emergency Department of a cancer center, the probabilities of successful resuscitation and being discharged alive are 43% and 17%, respectively.8 The mortality rate of cancer patients in intensive care is about 50%, which is similar to that of severely ill non-cancer patients.9 If a cancer patient has good performance status and is not expected to die soon, reluctance to resuscitate the patient or admit the patient to intensive care is unjustified. A non-end stage cancer patient in cardiopulmonary arrest should be resuscitated with the same level of intense effort as any noncancer patient. However, when cardiopulmonary arrest occurs as the expected final event, resuscitation is generally futile.
Oncologists should ensure that advance directives (medical power of attorney, living will, and out-of-hospital DNR orders) are discussed with cancer patients and their families. Many informed patients readily sign living wills or appoint health care proxies. Timely recommendation of DNR status may avoid unnecessary trauma to patients, futile efforts, wasted resources, and anguish for family members; this also provides time for open discussion to settle disagreements among the patient and family members.
When a cancer patient presents to a health care facility in impending or full cardiopulmonary arrest, the emergency physician may have never seen the patient before, and assessment of prognosis is difficult and often impossible. The decision to initiate or continue resuscitation should be based on a rapid assessment of the patient’s physical condition, a brief history of the events preceding the arrest, and the following factors: (1) duration of arrest, (2) initial cardiac rhythm, (3) rigor mortis or algor mortis, (4) type, stage, and prognosis of cancer, (5) history of cancer treatment and prospects for its success, (6) expressed directives of the patient or family, (7) comorbid conditions, (8) performance status and nutritional status, (9) potential quality of life if the patient survives, and (10) advanced age.
The fact that the patient was transported to an emergency center may indicate that death is unexpected, that the family has not yet accepted the patient’s grave prognosis, or that the patient or family is seeking relief of symptoms or suffering at the last moments of life. Demands for resuscitation may be motivated by denial of the terminal condition. A questionnaire-based study has found that most cancer patients want to be resuscitated despite poor survival rates, and that they want themselves and their next of kin to be involved in the decision-making process.10 In the absence of clear advance directive, resuscitation may be needed to give the family “closure” by knowing that “everything possible has been done.” However, resuscitation of patients with advanced refractory malignancies may be inappropriate when it will only prolong pain and suffering.
Special consideration in resuscitation of cancer patients
Most physicians and health care providers follow the resuscitation algorithms outlined in the advanced cardiac life support (ACLS) protocols (http://www.acls.net/aclsalg.htm).5 However, identification of specific causes of cardiopulmonary arrest may enable physicians to target efforts to reverse or control the specific causes. Carcinoid crisis is a good example of an uncommon but preventable and treatable cause of cardiopulmonary arrest in cancer patients. The crisis may be precipitated by anesthesia, biopsy, surgery, chemotherapy, or adrenergic drugs (e.g., dopamine and epinephrine). Affected patients may develop refractory hypotension, arrhythmias, and bronchospasm due to massive release of serotonin and other vasoactive peptides from the tumor. Carcinoid crisis can be aborted or treated with octreotide acetate, a somatostatin analog, 150–500 µg intravenously (IV).11 Cardiac tamponade is another example. If a patient has pulseless electrical activity due to tamponade by a malignant pericardial effusion, resuscitation will not succeed until the pressure on the cardiac chambers is relieved by pericardiocentesis.
Causes of cardiac arrest in cancer patients
In the general population, undiagnosed neoplasm is a rare cause of sudden death. In cancer patients, a review of causes of death found that 4% of patients died of cardiac problems and that 90% of these died from atherosclerosis-related ischemic heart disease.12 Most causes of cardiopulmonary arrests in cancer patients are related to cancer or antineoplastic therapy, rather than primary cardiac disease.
Tumor-related causes
Tumor-related cardiac problems are usually the result of pericardial involvement (e.g., neoplastic pericarditis and cardiac tamponade). Tumors can induce arrhythmias by secretion of hormone mediators (e.g., catecholamines by pheochromocytomas and serotonin by carcinoid tumors) or by direct mechanical irritation of the heart or pericardium. Arrhythmias associated with myocardial tumors, coronary obstruction by tumor, and massive tumor embolization have been reported to cause sudden cardiopulmonary arrest. Cardiac amyloidosis can also lead to intractable congestive heart failure, arrhythmias, conduction disturbances, and sudden death.13 Other tumor-related causes of arrest include hemorrhage, loss of ventilatory function, and organ failure.
Systemic therapy-related causes
Antineoplastic agents can cause complications (angina, myocardial infarction, congestive heart failure, hypotension, arrhythmia) leading to cardiopulmonary arrest.14 Doxorubicin may cause electrocardiographic and rhythm changes (mostly benign) in about 30% of patients15 and sudden cardiopulmonary arrest in almost 1% of patients.16 Some drugs (e.g., imatinib, trastuzumab) may interfere with myocardial remodeling after cytotoxic myocardial damage, causing cardiomyopathy and heart failure.15 High-dose cyclophosphamide may cause ventricular arrhythmia, cardiomyopathy, pericardial effusion, and cardiac arrest.17 Fluorouracil and capecitabine are associated with acute coronary vasospasm leading to angina and myocardial infarction; they have also been reported to cause acute cardiogenic shock. Hypotension, arrhythmia, and sudden death have been reported with cytokines (interleukin-2 [IL-2], interferons) and monoclonal antibodies.
Radiotherapy-related causes
Radiation can damage the pericardium and heart.18 Pericarditis may occur shortly or months to years after exposure of the chest to radiation. Radiotherapy can lead to valvular diseases, pericardial effusion, tamponade, pericardial fibrosis, or restrictive cardiomyopathy. The direct toxic effect of radiation can cause electrocardiographic changes, including T-wave abnormalities and atrial arrhythmias. Exposure of the heart to radiation is also associated with coronary artery problems (accelerated atherosclerosis, endarteritis, medial fibrosis, intimal proliferation), leading to myocardial infarction and sudden death.19
Arrhythmia
Arrhythmia is a common problem in cancer patients that needs emergency care. Sustained arrhythmia can lead to cardiopulmonary arrest and death; otherwise, the symptoms of intermittent arrhythmia can be subtle. The symptoms are primarily due to the hemodynamic effects. Significant signs and symptoms include isolated or recurrent loss of consciousness (syncope), light-headedness (dizziness), palpitation, chest pain, dyspnea, and acute neurologic deficits.
Sustained arrhythmia can be diagnosed electrographically readily. However, arrhythmia is often transient or intermittent, causing difficulty in diagnosis. An electrocardiographic rhythm strip or a brief period of continuous monitoring does not exclude latent and potentially serious rhythm disorders. When symptoms suggest arrhythmia, Holter monitoring for 24–48 h or event recorders (continuous loop, postevent, or real-time continuous) are indicated to capture the arrhythmia. Analysis of cardiac rhythm may be complicated in cancer patients because they often have exaggerated respiratory variations of the electrical axis and changes in mean QRS voltage that can be confused with heart rhythm irregularity. Such changes may be due to pleural or pericardial effusions, pulmonary surgery (pneumonectomy or lobectomy), or radiation-induced lung damage.
Primary arrhythmia
Primary arrhythmia arises from cardiac and pericardial structures. Common causes of primary arrhythmia in all patients include ischemic disease; increased intracardiac pressure and wall stress; congestive, hypertrophic, and infiltrative cardiomyopathy; and fibrosis. In cancer patients, the causes of primary arrhythmia are primary or metastatic intracardiac tumors, amyloid infiltration, myocarditis, pericarditis, pericardial constriction, and cardiomyopathy related to antineoplastic agents (especially anthracyclines and anti-HER2 therapy).14
Secondary arrhythmia
Secondary arrhythmia arises from toxic reactions to drugs; increased sympathetic states (severe anxiety, hyperthyroidism, pheochromocytomas, carcinoid tumors, etc.); abnormal electrolytes; and radiation-induced heart damage. Some cancer drugs are arrhythmogenic (Table 1).20–24 In addition to chemotherapy, antifungal agents, antiprotozoans, and antibiotics, which are commonly used to treat infectious complications in cancer patients, may prolong the QT interval [listed at http://www.qtdrugs.org (Arizona Center for Education and Research on Therapeutics)], potentially leading to arrhythmia.
Table 1 Antineoplastic drugs associated with cardiovascular side effects
| Pulmonary HTN | Systemic HTN | Ischemia | Reduction in LVEF | QT prolongation | VT/Sudden death | Bradycardia | AF/SVT | |
| Tyrosine kinase inhibitors | ||||||||
| Imatinib | Reported | 0.5–1.7% | ||||||
| Dasatinib | Reported | 2–4% | <1–3% | |||||
| Cabozantinib | 33% | Mean increase of QTcF by10–15 ms; No QTcF >500 ms | ||||||
| Lapatinib | 1.5–4% | 16% | ||||||
| Erlotinib | 2.3% | |||||||
| Nilotinib | Reported | 10–11% | 1–10%; QTcF > 500 ms in 1% | Reported | ||||
| Pazopanib | 40–42% | 8–11% | QTcF > 500 ms in 0.2–2% | Torsades de pointes in <1% | ||||
| Sorafenib | 9–43% | 2.7–3% | 12% | No large changes (i.e., <20 ms) | ||||
| Sunitinib | 5–47% | 2.7–27% | Dose-dependent | Torsades de pointes in 0.1% | ||||
| Vandetanib | 33% | QTcF > 500 ms in 1% | Reported | |||||
| Antibodies | ||||||||
| Bevacizumab | 4–35% | 0.6–1.5% | 1.7–3% | |||||
| Trastuzumab | 4% | 2–28% | ||||||
| Alkylating agents | ||||||||
| Cisplatin | Reported | Reported | Reported | Reported | Reported | |||
| Ifosfamide | 17% | Reported | ||||||
| Cyclophosphamide | Reported | 7–28% | Reported | |||||
| Busulfan | Reported | |||||||
| Mitomycin | Reported | |||||||
| Anthracyclines/anthraquinones | ||||||||
| Daunorubicin | Reported | |||||||
| Doxorubicin | 3–26% 5% at the cumulative dose of 400–450 mg/m2 | 6% | Reported | 2.2–10.3% | ||||
| Idarubicin | 5–18% | |||||||
| Epirubicin | 0.9–3.3% | |||||||
| Mitoxantrone | Reported | |||||||
| Antimetabolites | ||||||||
| Capecitabine | 3–9% | Cardiogenic shock reported | 2.1% | |||||
| 5-Fluorouracil | 1–68% | Cardiogenic shock reported | 4.2–6.5% | |||||
| Gemcitabine | 8.2% | |||||||
| Cytarabine | Reported | |||||||
| Clofarabine | 27% | |||||||
| Topoisomerase I inhibitors | ||||||||
| Irinotecan | Reported | |||||||
| Taxanes | ||||||||
| Paclitaxel | <1–5% | 0.26% | <0.1–31% | 0.18% | ||||
| Docetaxel | 1.7% | 2.3–8% | ||||||
| Vinca alkaloids | ||||||||
| Vincristine | Reported | |||||||
| Vinblastine | Reported | |||||||
| Miscellaneous | ||||||||
| IL-2 | Reported | 0.2% | 17.4% | |||||
| Arsenic trioxide | 26% | |||||||
| Vorinostat | 3.5–6% | |||||||
| Interferon-α | 1% | Reported | 1% | |||||
| Tretinoin (retinoic acid) | Reported | |||||||
| Bortezomib | 2–5% | |||||||
| Thalidomide | 0.12–55% | |||||||
| Pentostatin | Reported | |||||||
Abbreviations: AF, atrial fibrillation; HTN, hypertension; LVEF, left ventricular ejection fraction; QTcF, QT interval corrected by Fridericia’s formula; SVT, supraventricular tachycardia; VT, ventricular tachycardia.
Source: Data from Yeh et al. 20, Lenihan and Kowey 21, Guglin et al. 22, Yeh and Bickford 23, and Ewer and Ewer 24.
Treatment of arrhythmia
Treatment of arrhythmia should be based on both urgency and etiology. For hemodynamically stable arrhythmias of secondary origins, the primary treatment should focus on correcting metabolic derangements (particularly potassium, calcium, and magnesium) and discontinuing culprit drugs. Specific treatment to reverse the causative factor should be administered. When treatment aimed at controlling the cardiac rhythm is necessary, standard guidelines for management of arrhythmia may be followed.5 Commonly used IV antiarrhythmic drugs are listed in Table 2.
Table 2 Commonly used IV antiarrhythmic drugs
| Name | Class | Dosea | Indicationa |
| Adenosine | Nucleoside | 6 mg IV over <3 s followed by NS 20 mL bolus; second dose and third dose of 12 mg 2 min apart as needed | Narrow complex PSVT; PSVT due to AV node or sinus node reentry |
| Amiodarone | Class III antiarrhythmic | Cardiac arrest: 300 mg IVP; 150 mg IVP q 3–5 min up to 2.2 g/day | |
| Stable wide-complex tachycardia: 150 mg IV over 10 min; repeat q 10 min as needed; maintenance infusion 0.5 mg/min; up to 2.2 g/day | Supraventricular or ventricular tachyarrhythmias; control of rapid atrial tachyarrhythmia in patients with low LVEF when digoxin is ineffective | ||
| Atropine | Anticholinergic | 0.5–1 mg IVP q 3–5 min as needed, up to 0.04 mg/kg | Symptomatic sinus bradycardia; Mobitz type 1 AV block; asystole |
| Digoxin | Digitalis glycoside | Loading dose: 10–15 µg/kg lean body weight in divided doses | To slow ventricular response in A. fib. or A. flutter; PSVT |
| Diltiazem | Calcium channel blocker | 0.25 mg/kg IV over 2 min; second dose 0.35 mg/kg IV over 2 min in 15 min prn; maintenance: 5–15 mg/h by titration | To slow ventricular response in A. fib. or A. flutter; PSVT; to terminate AV nodal re-entrant tachycardia |
| Esmolol | β-Blocker | 0.5 mg/kg over 1 min; then infuse at 0.05 mg/kg/min; titrate up to maximum of 0.3 mg/kg/min | PSVT, A. fib. or A. flutter; Reduce incidence of VF in MI or USA |
| Ibutilide | Class III antiarrhythmic | 1 mg IV over 10 min; repeat in 10 min prn | SVT including A. fib. A. flutter; effective for conversion of A. fib. flutter of relatively brief duration |
| Isoproterenol | β-Agonist | Infuse 2–10 µg/min; titrate | Symptomatic bradycardia; torsades de pointes refractory to Mg; β-blocker overdose |
| Lidocaine | Local anesthetic | 1–1.5 mg/kg IVP; repeat 0.5–0.75 mg/kg IVP q 5–10 min up to total of 3 mg/kg prn; maintenance: 30–50 µg/kg/min IV | VT or VF; wide-complex tachycardia; significant ventricular ectopy; torsades de pointes |
| Metoprolol | β-Blocker | 5 mg slow IVP q 5 min up to a total dose of 15 mg | PSVT, A. fib., or A. flutter; Reduce incidence of VF in MI or USA |
| Procainamide hydrochloride | Class IA antiarrhythmic | 20–50 mg/min up to a total dose of 17 mg/kg | Recurrent VF or VT |
| Propranolol | β-Blocker | 0.1 mg/kg slow IVP in three divided doses 2–3 min apart | PSVT, A. fib., or A. flutter; Reduce incidence of VF in MI or USA |
| Quinidine gluconate | Class IA antiarrhythmic | Intermittent bolus doses of 80 mg every 5–10 min or 10 mg/min IV infusion up to 400 mg | Supraventricular and ventricular arrhythmias |
| Verapamil | Calcium channel blocker | 2.5–5 mg IV over 2 min; repeat q 15–30 min prn up to a total dose of 20 mg | PSVT, A. fib., or A. flutter |
Abbreviations: A. fib., atrial fibrillation; A. flutter, atrial flutter; AV, atrioventricular; IV, intravenous; IVP, intravenous push; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NS, normal saline; prn, as needed; PSVT, paroxysmal supraventricular tachycardia; USA, unstable angina; VF, ventricular fibrillation; VT, ventricular tachycardia.
a Source: From ACLS Provider Manual.5
Paroxysmal supraventricular tachycardia (SVT) may be converted back into sinus rhythm in a considerable proportion of cases by vagal maneuvers. Adenosine administered as one or two doses of rapidly injected boluses under electrocardiographic monitoring is frequently effective in restoring sinus heart rhythm. Adenosine is also used to determine the mechanism of the arrhythmia when the diagnosis is unclear on electrocardiograms.
Stable secondary arrhythmia is unlikely to deteriorate into a life-threatening catastrophe. Frequently, secondary arrhythmia presents as ventricular ectopy (sometimes in bigeminy, trigeminy, or other coupled patterns), or supraventricular ectopy (often as intermittent or sustained SVT). Isolated premature ventricular complexes do not require any treatment. Complex forms of ventricular ectopy are often controlled by β-adrenergic blockers. Amiodarone should be considered for patients with a low left ventricular ejection fraction, but with caution for patients with hepatic insufficiency or underlying thyroid diseases. Rarely, amiodarone can cause hypotension, bradycardia, and QT prolongation that may precipitate torsades de pointes. Except for β-adrenergic blockers, many antiarrhythmic drugs, especially types 1A, 1C, and 3, are potentially proarrhythmic.25 Cardiac monitoring during the initiation of antiarrhythmic therapy should be considered because cancer patients may have an increased susceptibility to proarrhythmic effects due to metabolic derangements and concomitant use of other QT-prolonging drugs.
SVT is the most common arrhythmia in cancer patients. Although pharmacological agents are used for sustained SVT with stable hemodynamics, elective synchronized cardioversion under conscious sedation should be considered early and planned appropriately. The initial energy level for synchronized cardioversion recommended by the American Heart Association is 100 J, but an initial shock with an energy level of 200 J has been recommended by others for the conversion of atrial fibrillation.26 Higher energy levels for cardioversion are appropriate when cancer patients have concomitant effusions or are significantly overweight. If sinus rhythm can be restored within 48 h of the onset of SVT, anticoagulation therapy may be avoided. However, the time of onset of arrhythmia is not always clear. Intracardiac thrombosis may be excluded by transesophageal echocardiography. In lack of clear evidence for the time of onset, the patient should be anticoagulated prior to cardioversion.
Arrhythmias of structural origin in cancer patients are more difficult to control than arrhythmias of metabolic etiology. In the emergency setting, the therapeutic goals are stabilization of hemodynamics and respiratory status, discovery of correctable pathologic conditions, and control of symptoms. Depending on the etiology of the arrhythmia, emergent consultation with cardiologists and emergent diagnostic or interventional procedures may be required.
Patients with unstable arrhythmia should be treated with aggressive pharmacological or electrical interventions. The interventions should follow established algorithms such as those by the American Heart Association.27 These interventions include administration of a vasopressor, such as vasopressin or epinephrine (if required); administration of antiarrhythmic drugs such as amiodarone, lidocaine, and procainamide; electrical cardioversion or defibrillation; airway management; ventilation with oxygen; administration of IV fluid; and chest compression (if required). Emergency treatment of torsades de pointes varies from the standard algorithms for ventricular tachycardia; it entails expedient use of IV magnesium sulfate, electrical overdrive pacing, pharmacological overdrive with isoproterenol, or administration of phenytoin or lidocaine.
Tumor lysis syndrome
Tumor lysis syndrome (TLS) consists of severe hyperphosphatemia, hyperkalemia, hyperuricemia, azotemia, hypocalcemia, and metabolic acidosis (out of proportion to renal insufficiency) due to the massive release of cell contents and degradation products of dead tumor cells into the bloodstream.28 TLS can occur spontaneously, but it usually occurs within 72 h after chemotherapy in patients with leukemia and lymphoma, but new therapeutic regimens may alter the timing of onset. TLS can also occur in patients with nonhematologic malignancies, including small cell carcinomas, nonsmall cell lung cancer, breast cancer, and ovarian cancer.
The symptoms of TLS are nonspecific. Common symptoms include nausea, vomiting, cloudy urine, weakness, fatigue, and arthralgia. Other signs and symptoms related to metabolic and electrolyte abnormalities include neuromuscular irritability, seizures, muscle weakness, and arrhythmia. Arrhythmia may cause sudden death in patients with TLS.29 Precipitation of uric acid in the renal tubules may lead to nephropathy and acute renal failure.30 The acute cause of death in TLS is arrhythmia secondary to severe electrolyte abnormalities (especially hyperkalemia) and renal failure. Early recognition of metabolic abnormalities and prompt treatment can avoid fatal outcomes.
Factors associated with increased risk of TLS include the type of malignancy (e.g., acute lymphocytic leukemia, acute myeloid leukemia with white blood cell count > 75,000/μL, Burkitt’s lymphoma), responsiveness to therapy, rapid malignant cell turnover, and large tumor burden.31 Other risk factors are preexisting renal insufficiency, acute renal failure developing shortly after the treatment, and poor response to hydration. Pretreatment serum lactate dehydrogenase levels, which tend to correlate with tumor bulk in lymphoma or lymphocytic leukemia, can predict the development of posttreatment azotemia, but pretreatment hyperuricemia is not predictive. A predictive scoring system for TLS has been proposed based on data from acute myelocytic leukemia patients undergoing induction therapy.32, 33 The score may potentially be used in a risk-based prophylaxis for TLS. Preventive measures should be started early in patients at risk. Aggressive hydration with IV crystalloid fluid up to 3 L/m2/day may maintain a urine output >100 mL/h with or without diuretics. The xanthine oxidase inhibitor allopurinol (100–300 mg/day orally) may prevent severe hyperuricemia. The role of febuxostat, a new xanthine oxidase inhibitor, remains to be studied.
The diagnosis of TLS requires a high level of suspicion because there are few signs or symptoms in the early stage. Routine uric acid and electrolyte screening (including measurement of calcium and phosphorus levels) is indicated in patients with high tumor bulk or hematologic malignancies. The diagnosis of TLS may be based on the Cairo–Bishop definition.31, 34 Once diagnosed, patients with severe TLS should have continuous monitoring of hemodynamic and electrocardiographic parameters in intensive care. The allopurinol dose may be increased up to 900 mg/day. Rasburicase, a recombinant urate oxidase that converts uric acid to allantoin, is highly efficacious in reducing uric acid level. Rasburicase (150–200 µg/kg IV daily or one-time dosing with a rescue dose as needed) may be used to prevent or treat urate nephropathy.35 Increased IV fluid hydration may be coupled with diuresis using loop diuretics (e.g., furosemide, 20–200 mg IV every 4–6 h) and acetazolamide (250–500 mg IV daily). Urinary alkalinization by sodium bicarbonate or acetate IV infusion to increase the solubility of urate in urine should only be considered in cases of severe hyperuricemia when rasburicase is not available. Frequent electrolyte measurements (every 4–6 h) may be required. Hyperkalemia should be treated with insulin plus dextrose, calcium, and bicarbonate IV along with oral potassium ion-exchange resins (sodium polystyrene sulfonate). In hyperphosphatemic patients with hypocalcemia, the addition of an oral calcium-based compound (e.g., calcium acetate or calcium carbonate) will reduce phosphate absorption and enhance calcium absorption. IV calcium infusion can potentially cause calcium phosphate precipitation in the presence of severe hyperphosphatemia and should be used cautiously. Dialysis may be required for patients with symptomatic hypocalcemia and a serum phosphorus level >3.3 mmol/L (>10.2 mg/dL). Other indications for dialysis include persistent or refractory azotemia, hyperkalemia, hyperuricemia, oliguria, anuria despite diuretic use, acidemia, and volume overload. Prompt dialysis should be instituted with continued monitoring until biochemical abnormalities resolve. Hemodialysis is the most common mode of dialysis; prolonged hemodialysis sessions, continuous arteriovenous hemodialysis, continuous veno-venous hemofiltration, and continuous renal replacement therapy at a high dialysate or replacement fluid flow rate (>3 L/h) are alternative methods.
Pericardial tamponade
Pericardial tamponade occurs when a pericardial effusion impairs hemodynamics. Accumulation of excess fluid in the pericardial space in cancer patients is due to obstruction of lymphatic drainage and/or excess fluid secretion from tumor nodules on pericardial surfaces. Mesothelioma is the most common malignancy that arises from the pericardium. Carcinoma of the lung and malignant thymoma may involve the pericardium by direct extension. More frequently, malignancies arrive at the pericardium by retrograde lymphangitic spread or hematogenous dissemination. Melanoma is the malignancy most likely to metastasize to the heart. Lymphomas, leukemias, and gastrointestinal neoplasms may also cause pericardial effusions.36 Cytologic examination of pericardial fluid reveals metastatic disease in 70–80% of cancer patients with pericardial effusion. Nonmalignant causes of pericardial tamponade include pericardial abscess, Candida pericarditis, and complications of central venous catheterization.
Malignant pericardial effusion usually occurs in advanced malignancy and is associated with poor prognosis (median survival time: about 6 months; 1-year survival rate: 28%).37 More than two-thirds of patients with malignant pericardial effusion are asymptomatic. In symptomatic patients, common complaints are shortness of breath, dyspnea on exertion, chest pain, orthopnea, and general weakness. Findings on physical examination may vary from normal to hemodynamic collapse. Tachycardia, hypotension, jugular venous distention, organomegaly, and edema may indicate compromised cardiac output. The classic findings of cardiac tamponade are determined by both the quantity of pericardial fluid and the rapidity of fluid accumulation. Pulsus paradoxus, an exaggeration of the physiological decrease in systolic blood pressure with inspiration, is a classic but nonspecific finding of cardiac tamponade because it is seen also in patients with lung cancer, significant lung disease, or cor pulmonale.
Diagnosis of pericardial tamponade usually requires additional testing. Low QRS voltage and electrical alternans in electrocardiographs are suggestive findings. Chest radiographs may reveal widening of the mediastinum and cardiac silhouette (Figure 2a, b). Computed tomography (CT) or magnetic resonance (MR) imaging studies frequently detect pericardial effusions as an incidental finding. These studies provide information on the location (loculated or not) and size of pericardial effusions but do not adequately assess the hemodynamic significance. Two-dimensional echocardiography is the most useful test for diagnosing pericardial effusion and evaluating its hemodynamic significance, that is, the presence of cardiac tamponade. Collapse or compression of the right atrium, diastolic collapse of the right ventricle, and cardiac “rocking” (side-to-side or front-to-back movement) are often observed in cardiac tamponade. Alterations in the respiratory variation of flow across the mitral valve as measured by Doppler shift are also helpful in evaluating the hemodynamics.
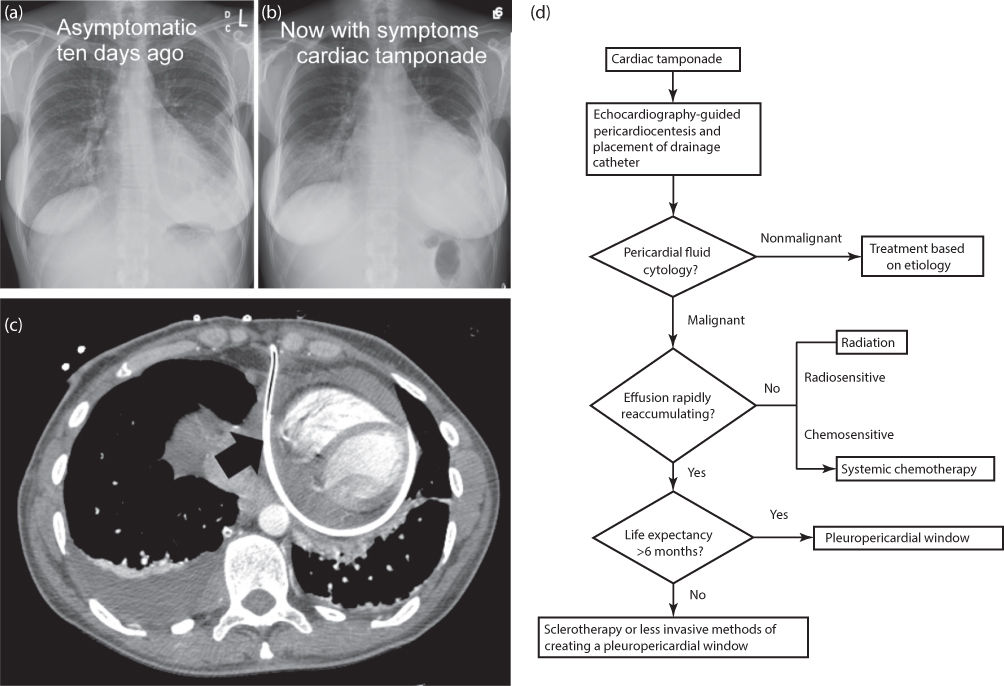
Figure 2 Chest imaging of patients with pericardial tamponade and management algorithm. Widening of mediastinum and cardiac silhouette is evident (b) when compared with a prior chest radiograph (a). Chest CT of a different patient with pericardial tamponade shows the presence of an indwelling drainage catheter (c). An algorithm for management of pericardial effusion is shown (d).
Initial management of malignant pericardial effusion depends on the hemodynamic stability. A scoring system may guide the decision for urgent pericardiocentesis.38 In patients with hemodynamic compromise, ultrasound-guided pericardiocentesis, with placement of a drainage catheter into the pericardial space (Figure 2c), may be performed emergently in the emergency center or intensive care unit (Figure 2d). Complications are rare and may include massive pericardial bleeding and pneumothorax. Pericardial fluid can be drained from the catheter, and the catheter can stay until <50 mL/day of fluid is drained. Fibrinolytic agents may be used to unclog the catheter to facilitate drainage and avoid repeat pericardiocentesis or replacement of the catheter.39 However, pericardial fluid will usually reaccumulate after removal of the catheter.
Long-term management of malignant pericardial effusion focuses on preventing reaccumulation of fluid, which occurs in >50% of patients. Because the long-term survival for most patients with malignant pericardial effusion is limited, an effective therapy with limited discomfort and risk to the patient should be employed. Creation of a pleuropericardial window using a variety of approaches can avoid repeated pericardiocentesis. This surgical procedure is usually done in an operating room, but it can be performed in a hospital room or intensive care unit using local anesthesia. The use of a percutaneous intrapericardial balloon catheter to create a pleuropericardial window has had some success.40 A laparoscopic transdiaphragmatic approach to create a pericardioperitoneal shunt has also been described.41 In stable patients, systemic chemotherapy, pericardial radioactive colloid, or thoracic external-beam irradiation may be used for tumors that are sensitive to these treatment modalities. Additional radiotherapy should be avoided in patients with significant prior exposure of the heart to radiation. Local application of cytotoxic agents or sclerosing agents to the pericardium can prevent fluid reaccumulation in many patients,42 but sclerotherapy can be very painful.
Acute hemorrhage
Acute gastrointestinal bleeding and genitourinary bleeding are discussed in other chapters. Hemoptysis, which can rapidly compromise respiratory function, will be discussed later in this chapter. This section will cover some less frequent but serious bleeding events: carotid arterial rupture, splenic rupture, and retroperitoneal hemorrhage.
The manifestations of acute hemorrhage depend on the rate and the site of bleeding. In most cases, the site of bleeding is obvious, but sometimes bleeding can be internal and difficult to diagnose. Signs and symptoms of hypovolemia and hypoperfusion include tachycardia, hypotension, oliguria, and depressed mental status. Very often, diagnostic imaging studies or procedures, such as CT scans, ultrasonography, arteriography, or endoscopy, are necessary to diagnose internal bleeding.
The primary management objectives for acute hemorrhage are to rapidly identify the bleeding source and achieve hemostasis. In the acute setting, direct pressure to compress the bleeding vessel or site should be applied whenever feasible while the cardiopulmonary status is assessed expeditiously. IV fluid resuscitation is vital in maintaining intravascular volume, cardiac output, and adequate vital organ perfusion. Isotonic crystalloid fluids (normal saline, lactated Ringer’s solution, PlasmaLyte, etc.) should be used as first-line agents because colloids (e.g., gelatins, dextrans, hydroxyethyl starches, albumin) have not been proven to improve survival.43 Coagulopathy or thrombocytopenia should be corrected immediately by transfusion of blood products. The decision to transfuse red blood cells depends on the hematocrit, hemodynamic stability, persistence of hemorrhage, estimated blood loss, and comorbid diseases (e.g., coronary artery disease and cerebrovascular disease). Typed and cross-matched red blood cells are preferred, but noncross-matched type-specific blood or type-O blood may have to be used in life-threatening cases. Specific therapeutic procedures to control bleeding, such as embolization, balloon tamponade, or surgery, should be performed in a timely manner.
Carotid artery rupture
Most cases of carotid artery “blowout” occur in patients with head and neck cancers. Carotid blowout syndrome may be caused by direct tumor invasion or erosion into the carotid artery or by complications of cancer treatment, for example, postsurgical wound infection, postradiation necrosis, or orocutaneous fistula. It usually occurs as a sudden and massive arterial spurting. Occasionally, ominous minor and transient bleeding (sentinel bleeds) herald the massive blowout. In some cases, bleeding through a fistula into the esophagus or trachea may manifest as massive hematemesis or hemoptysis. Without prompt management, the patient’s condition will rapidly deteriorate to hypotension, hypovolemic shock, loss of consciousness, and death.
Hemostasis is of utmost importance. As neck vessels are accessible to direct manual compression, continuous firm compression should be applied at the site of the carotid artery rupture until the patient arrives at the operating room for surgical treatment. Crystalloid IV fluid resuscitation, prompt transfusion of blood products, and administration of vasopressors should be performed to maintain perfusion of vital organs. Carotid artery rupture has limited surgical options, and surgical ligation of the bleeding carotid artery is associated with high morbidity (25% of patients have neurologic sequelae) and high mortality (40%).44 Endovascular treatment with vessel sacrifice (embolization or balloon occlusion) or stent placement (covered stent) has become major treatment options.45, 46
Splenic rupture
The spleen is fragile and vulnerable to rupture from trauma. In cancer patients, spontaneous splenic rupture is relatively rare and is associated with acute leukemia, non-Hodgkin’s lymphoma, chronic myelogenous leukemia, hairy cell leukemia, and Hodgkin’s lymphoma. Metastases to the spleen in patients with solid tumors such as gastric, prostate, and lung cancer can also cause rupture. The mechanism of spontaneous splenic rupture is not clear. Minor trauma to the spleen may contribute in some cases. Other contributing factors include splenomegaly, infiltration of the splenic capsule by malignant cells, splenic infarction, thrombocytopenia, coagulopathy, anticoagulation therapy, and disseminated intravascular coagulation.
The typical clinical presentation of splenic rupture involves pain in the left shoulder or abdomen (left upper quadrant), tachycardia, and hypotension. The severity of the signs and symptoms may depend on the extent of bleeding. Diagnostic peritoneal lavage is rarely used in nontraumatic cases; thus, the definitive diagnosis of splenic rupture relies on imaging studies. Contrast-enhanced CT is the diagnostic study of choice; ultrasonography can be performed at bedside for hemodynamically unstable patients to diagnose splenic rupture.47
For patients with splenic rupture and hematologic malignancies, prompt splenectomy is necessary because the mortality rate for these patients is extremely high without surgery. In selected patients with contraindications to surgery, selective arterial embolization of the ruptured site may stop the bleeding.48 Other supportive treatments are IV fluid, supplemental oxygen, pain medications, blood transfusion, and correction of thrombocytopenia and coagulopathy.
Retroperitoneal hemorrhage
Damage to retroperitoneal organs or structures may cause retroperitoneal hemorrhage. Malignancies rarely cause spontaneous retroperitoneal hemorrhage; in such cases, the culprit is usually renal cell carcinoma or adrenal gland neoplasm (primary or metastatic). Anticoagulation, thrombocytopenia, and coagulopathy are predisposing factors. Retroperitoneal or intraperitoneal invasive procedures and placement of a central venous catheter through a femoral vessel can also cause severe retroperitoneal hemorrhage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








