Virus
Cancer sites with sufficient evidence
Cancer sites with limited evidence
Epstein–Barr virus (EBV)
Nasopharyngeal carcinoma, Burkitt’s lymphoma, immune suppression-related non-Hodgkin lymphoma, extranodal NK/T cell lymphoma (nasal type), Hodgkin’s lymphoma
Gastric carcinoma, lympho-epithelioma-like carcinoma
Hepatitis B virus (HBV)
Hepatocellular carcinoma
Cholangiocarcinoma, non-Hodgkin lymphoma
Hepatitis C virus (HCV)
Hepatocellular carcinoma, non-Hodgkin lymphoma
Cholangiocarcinoma
Human immunodeficiency virus, type 1 (HIV-1)
Kaposi’s sarcoma, non-Hodgkin lymphoma, Hodgkin’s lymphoma, cancers of the cervix, anus, and conjunctiva
Cancers of the vulva, vagina and penis, non-melanoma skin cancer, hepatocellular carcinoma
Human papillomavirus type 16 (HPV-16)
Cancers of the cervix, vulva, vagina, penis, anus, oral cavity, oropharynx, and tonsil
Cancer of the larynx
Human papillomavirus type 18, 31, 33,35, 39, 45, 51, 52, 56, 58, 59 (HPV-18, 31, 33,35, 39, 45, 51, 52, 56, 58, and 59)
Cancer of the cervix
Human papillomavirus type 26, 30, 34, 53, 66, 67, 68, 69, 70, 73, 82, 85, 97 (HPV- 26, 30, 34, 53, 66, 67, 68, 69, 70, 73, 82, 85, and 97)
Cancer of the cervix
Human T cell lymphotrophic virus, type-1 (HTLV-1)
Adult T cell leukemia and lymphoma
Kaposi’s sarcoma herpes virus (KSHV)
Kaposi’s sarcoma, primary effusion lymphoma
Multicentric Castleman’s disease
There is sufficient evidence to conclude that EBV causes nasopharyngeal carcinoma, Burkitt’s lymphoma, immune suppression-related non-Hodgkin lymphoma, extranodal NK/T cell lymphoma (nasal type), and Hodgkin’s lymphoma in humans. The evidence for EBV-caused gastric carcinoma and lympho-epithelioma-like carcinoma is limited. HBV and HCV cause hepatocellular carcinoma with sufficient evidence. The evidence for HCV-caused non-Hodgkin lymphoma, especially B-cell lymphoma, is sufficient, while the evidence for HBV-caused non-Hodgkin lymphoma is limited. There is also limited evidence to conclude that HBV and HCV cause cholangiocarcinoma. The evidence to conclude that HIV-1 causes Kaposi’s sarcoma, non-Hodgkin lymphoma, Hodgkin’s lymphoma, and cancers of the cervix, anus, and conjunctiva is sufficient. But the evidence for HIV-1 to cause cancers of the vulva, vagina, penis, non-melanoma skin cancer, and hepatocellular carcinoma is limited.
There is sufficient evidence to conclude that HPV-16 causes cancers of the cervix, vulva, vagina, penis, anus, oral cavity, oropharynx, and tonsil; but the evidence for HPV-16 to cause cancer of the larynx is limited. Cervical cancer is caused by several types of HPV including HPV-18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59. The evidence for HPV-26, 30, 34, 53, 66, 67, 68, 69, 70, 73, 82, 85, and 97 to cause cervical cancer is limited. HTLV-1 causes adult T cell leukemia and lymphoma with sufficient evidence. There is sufficient evidence to conclude KSHV causes Kaposi’s sarcoma and primary effusion lymphoma, but the evidence for KSHV to cause multicentric Castleman’s disease is limited.
The proportion of cancers caused by infectious agents was recently estimated to be more than 20 % (IARC 2009). The identification of new cancer sites attributed to these agents means that more cancers are potentially preventable. This chapter will review mainly the epidemiology of oncogenic viruses and their associated cancers.
2 Prevalence of Oncogenic Virus Infection in the World
EBV is highly prevalent throughout the world with more than 90 % adults infected with EBV even in the remote populations (IARC 2009). The estimated number of persons infected with EBV is more than 5.5 billion. The age at primary infection of EBV varies significantly in the world. People live in overcrowded conditions with poor sanitation have a younger age at primary infection than those live in better environments. Two major types of EBV have been identified and differ in geographical distribution with EBV-2 more common in Africa and homosexual men. The role of specific EBV types in the development of difference cancers remains to be elucidated. As EBV infection is ubiquitous, the specific geographical distribution of EBV-related malignancies including endemic Burkitt lymphoma and nasopharyngeal carcinoma is more likely attributable to the variation in the distributions of other cofactors which may activate EBV replication.
Figure 1 shows the geographical variation in the prevalence of oncogenic viruses in the world. HBV infects more than 2.0 billion people in the world and more than 300 million of them are chronic HBV carriers (IARC 2009). There is a wide variation of chronic HBV infection in the world1 as shown in Fig. 1a. Approximately 45 %, 43 %, and 12 % of the world population live in areas where the endemicity of chronic HBV infection is high (seroprevalence of hepatitis B surface antigen >8 %), medium (2–7 %), and low (<2 %). The prevalence is highest in sub-Saharan Africa, the Amazon Basin, China, Korea, Taiwan, and several countries in Southeast Asia. In areas of high endemicity, the lifetime risk of HBV infection is more than 60 % with most infections acquired from perinatal and child-to-child transmission, when the risk of becoming chronic infection is greatest. Perinatal (vertical) transmission is predominant in China, Korea, and Taiwan where the seroprevalence of HBeAg in pregnant women is high, while child-to-child (horizontal) transmission is common in sub-Saharan Africa where HBeAg seroprevalence is low in mothers. In areas of medium endemicity, mixed HBV transmission patterns occur in infancy, early childhood, adolescence, and adulthood. In the low endemicity areas, most HBV infections occur in adolescents and young adults through injection drug use, male homosexuality, health care practice, and regular transfusion or hemodialysis.
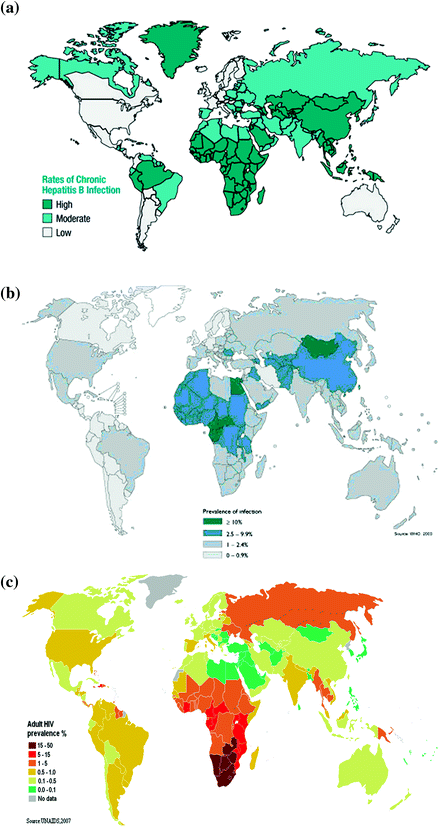
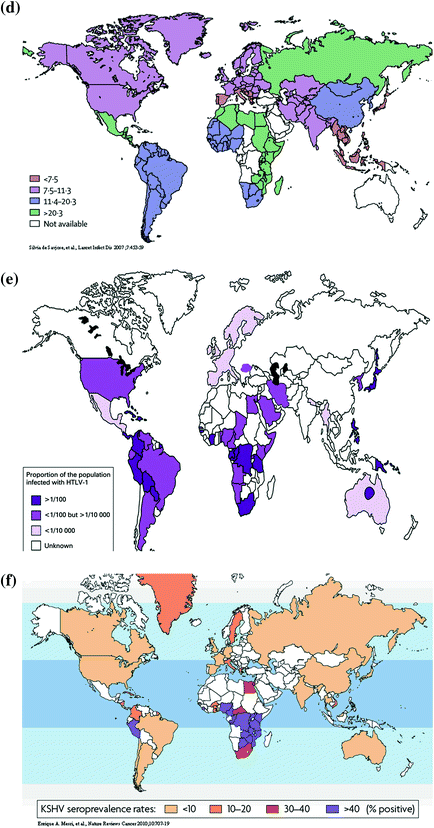


Fig. 1
Estimated prevalence (per 100) of Group 1 oncogenic viruses in the world. a HBV, b HCV, c HIV-1, d HPV, e HTLV-1, and f KSHV
In addition to the striking geographical variation in seroprevalence of HBsAg in the world, the distribution of eight genotypes of HBV varies significantly in different countries (IARC 2009). Genotype A is prevalent in Europe, Africa, and North America; genotypes B and C are prevalent in East and Southeast Asia; Genotype D is predominant in South Asia, Middle East, and Mediterranean areas; genotype E is limited to West Africa; genotypes F and G are found in Central and South America; and genotype H is observed in Central America.
HCV infects around 150 million people in the world showing an estimated prevalence of 2.2 % (IARC 2009) with a wide variation in different regions as shown in Fig. 1b. The estimates of HCV infection (seroprevalence of antibodies against HCV) range from <0.1 % in the United Kingdom and Scandinavia to 15–20 % in Egypt (Alter 2007). The high prevalence of HCV infection was observed in Mongolia, northern Africa, Pakistan, China, southern Italy, and some areas in Japan. There are at least six major genotypes of HCV have been identified. There is a wide variation in geographical distribution of HCV genotype in the world. The response to antiviral therapy also varies by HCV genotype. It is better in patients infected with genotype 2 or 3 than those with genotype 1 or 4.
HCV have two major transmission routes including injection drug use and iatrogenic exposures through transfusion, transplantation, and unsafe therapeutic injection. While iatrogenic transmission of HCV has been reduced after 1990 in developed countries such as Japan and Italy, it remains frequent in low-resource countries where disposable needles tend to be reused. Injection drug use is the most important transmission route for newly acquired HCV infection in developed countries. Transmission of HCV through perinatal, sexual, and accidental needle-stick exposures is less efficiently than iatrogenic exposure and injection drug use.
HIV infects estimated 34 million people in the world at the end of 2010 (IARC 2009).2 An estimated 0.8 % of adults aged 15–49 years worldwide are living with HIV, and the burden varies considerably between countries and regions as shown in Fig. 1c. Sub-Saharan Africa remains most severely affected with a prevalence of 4.9 %. Although the prevalence of HIV infection is nearly 25 times higher in sub-Saharan Africa than in Asia, almost 5 million people are living with HIV in South, Southeast, and East Asia combined. After sub-Saharan Africa, regions most heavily affected are the Caribbean and Eastern Europe and Central Asia, where 1.0 % of adults were living with HIV in 2011. There were 2.5 million people including 0.39 million children were newly infected with HIV in 2011. Since 2001, annual HIV incidence has fallen in 33 countries, 22 of them in sub-Saharan Africa. However, incidence is accelerating again in Eastern Europe and Central Asia after having slowed in the early 2000s, and new infections are on the rise in the Middle East and North Africa.
HIV-1 infection is transmitted through three major routes: sexual intercourse, blood contact, and mother-to-child transmission. The HIV-1 infectivity is determined by the interaction of three factors of agent, host, and environments. The probability of HIV-1 transmission is highest for blood transfusion, followed by mother-to-child transmission, needle sharing, man-to-man sexual transmission, and lowest for woman-to-man sexual transmission.
HPV infection is very prevalent in most sexually active individuals will acquire at least one genotype of anogenital HPV infection during their lifetime (IARC 2009). The estimated oncogenic HPV DNA point prevalence has been reported as high as 10 % in a meta-analysis of 157,879 women with normal cytology, giving an estimate of 600 million people being infected (de Sanjose et al. 2007). The point prevalence was highest (20–30 %) in Africa, East Europe, and Latin America; and lowest (6–7 %) in southern and western Europe and Southeast Asia demonstrating a striking geographical variation as shown in Fig. 1d. The estimated point prevalence is highly dynamic because both incidence and clearance rates are high.
Among 13 oncogenic HPV types, the most prevalent types include 16, 18, 31, 33, 35, 45, 52, and 58. HPV 16 is the most common type in all regions with prevalence ranging 2.3–3.5 %. HPV infections are transmitted through direct skin-to-skin or skin-to-mucosa contact. Anogenital HPV types spread mainly through sexual transmission in teenagers and young adults. Non-sexual routes including perinatal and iatrogenic transmissions account for a minority of HPV infections.
HTLV-1 infects estimated 15–20 million people in the world (IARC 2009). HTLV-1 infection is characterized by the micro-epidemic hotspots surrounded by low prevalence areas as shown Fig. 1e (Proietti et al. 2005). The HTLV-1 infection prevalence ranges from <0.1 % in China, Korea, and Taiwan to 20 % in Kyushu and Okinawa of Japan. The regions of high endemicity include southwestern Japan, parts of sub-Saharan Africa, the Caribbean Islands, and South Africa. HTLV-1 has three major transmission routes: vertical transmission, sexual transmission, and parenteral transmission. Vertical transmission through breast-feeding has a high efficiency to result in mother-to-child infection. However, in utero infectivity is low due to limited trafficking of HTLV-1-infected lymphocytes across placenta. The efficiency of sexual transmission of HTLV-1 depends on the proviral load and use of condom. Parenteral transmission through transfusion is significantly reducing due to the sensitive serological examination of blood products. Needle sharing associated with injection drug use is another parenteral route for HTLV-1 transmission.
Infection prevalence of KSHV determined by serological tests varies significantly in the world (Dukers and Rezza 2003) as shown in Fig. 1f. It ranges from 2–3 % in northern Europe to 82 % in Congo (IARC 2009). The prevalence is generally low (<10 %) in northern Europe, the USA, and Asia, elevated in Mediterranean region (10–30 %) and high in sub-Saharan Africa (>50 %). The KSHV is primarily transmitted via saliva. In the countries where KSHV prevalence is high, the infection occurs during childhood and increases with age. The transmission of KSHV among homosexual men is also via saliva. KSHV may also be transmitted with a low efficiency through prolonged injection drug use, blood transfusion, and organ transplantation.
3 Incidence of Some Virus-caused Cancers in the World
The world maps of age-adjusted incidence rates of some oncogenic virus-related cancers are shown in Fig. 2. The age-adjusted incidence rates of nasopharyngeal cancer range from <0.1 to 8.05 per 100,000 as shown in Fig. 2a. The highest incidence was observed in southern China, Southeast Asia, and sub-Saharan Africa, and the lowest incidence in Europe, western Africa, and Central America. Chinese ethnicity in different cancer registries has the highest incidence of nasopharyngeal cancer. As EBV infection is ubiquitous in humans, the uniquely high incidence of nasopharyngeal carcinoma suggesting Chinese lifestyles or genetic susceptibility may play an important role in the development of nasopharyngeal cancer.
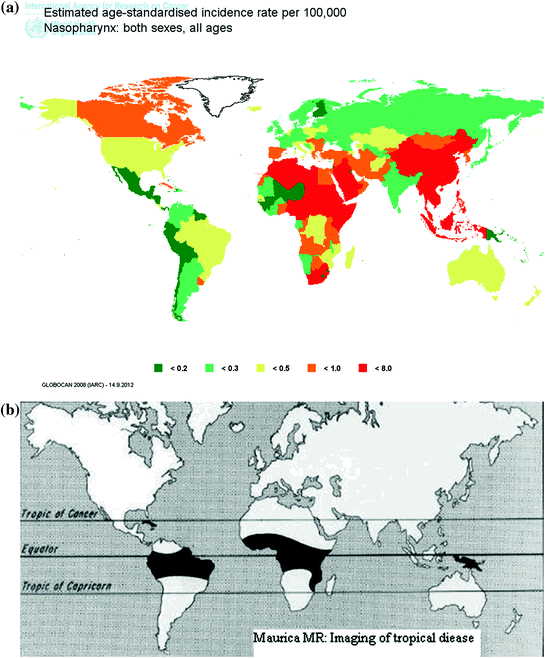
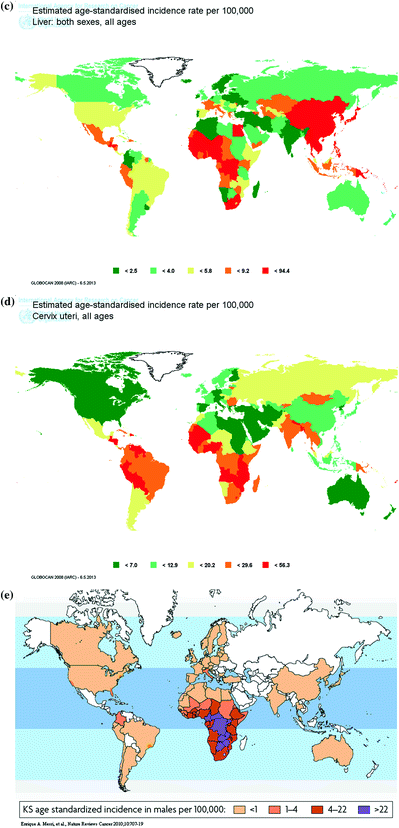


Fig. 2
Age-standardized incidence rate (per 100,000) of virus-caused cancers in the world. a Nasopharynx, b Burkitt lymphoma c Liver, d Cervix uteri, and e Kaposi’s sarcoma
The age-adjusted incidence rates of Burkitt lymphoma are shown in Fig. 2b. Central Africa, equatorial South America, Papua New Guinea, and Caribbean countries are endemic for Burkitt lymphoma, but the incidence rate of Burkitt Lymphoma is relatively low in other countries. As EBV infection is ubiquitous in humans, the extraordinarily high endemicity of Burkitt lymphoma in Africa suggesting local environments or genetic susceptibility may play an important role in the development of endemic Burkitt lymphoma.
The age-adjusted incidence rates of liver cancer range from 0.70 to 94.4 per 100,000 as shown in Fig. 2c. The highest incidence was observed in East Asia, Southeast Asia, Egypt, and sub-Saharan Africa, and the lowest incidence in Europe, Middle East, Australia, New Zealand, and Canada. The geographical variation in liver cancer incidence is consistent with that of seroprevalence of HBV and HCV.
The age-adjusted incidence rates of cervical cancer range from 2.14 to 56.29 per 100,000 as shown in Fig. 2d. The highest incidence was observed in Latin America, South Asia, and sub-Saharan Africa, and the lowest incidence in Europe, North America, Australia, New Zealand, and Middle East. The geographical variation in cervical cancer incidence is consistent with that of seroprevalence of oncogenic HPV.
The age-adjusted incidence rates of Kaposi’s sarcoma range from <1.0 to 30 per 100,000 as shown in Fig. 2e. The highest incidence was observed in sub-Saharan Africa and the lowest incidence in Europe, Australia, North America, and East Asia. The geographical variation in Kaposi’s sarcoma incidence is consistent with that of seroprevalence of KSHV.
4 Carcinogenic Mechanisms of Oncogenic Viruses
There are three major mechanisms of carcinogenesis for seven Group 1 oncogenic viruses as shown in Table 2. They are defined as direct, indirect through chronic inflammation, and indirect through immune suppression (IARC 2009). The direct carcinogens include EBV, HPV, HTLV-1 and KSHV; the indirect carcinogens through chronic inflammation include HBV and HCV; and the indirect carcinogen through immune suppression is HIV-1.
Table 2
Established carcinogenic mechanisms of oncogenic viruses
Mechanism | Group 1 virus (carcinogenic properties) |
|---|---|
Direct | EBV (cell proliferation, inhibition of apoptosis, genomic instability, cell migration) |
HPV (immortalization, genomic instability, inhibition of DNA damage response, anti-apoptotic activity) | |
HTLV-1 (immortalization and transformation of T cells) | |
KSHV (cell proliferation, inhibition of apoptosis, genomic instability, cell migration) | |
Indirect through chronic inflammation | HBV (inflammation, liver cirrhosis, chronic hepatitis) |
HCV (inflammation, liver cirrhosis, liver fibrosis) | |
Indirect through immune suppression | HIV-1 (immunosuppression) |
Direct oncogenic viruses have following characteristics: (1) The entire or partial viral genome can usually be detected in each cancer cell. (2) The virus can immortalize after the growth of target cells in vitro. (3) The virus expresses several oncogenes that interact with cellular proteins to disrupt cell-cycle checkpoints, inhibit apoptosis, and DNA damage response, cause genomic instability, and induce cell immortalization, transformation, and migration.
Both HBV and HCV cause hepatocellular carcinoma through chronic inflammation, which leads to the production of chemokines, cytokines, and prostaglandins secreted by infected cells and/or inflammatory cells. The chronic inflammation also leads to the production of reactive oxidative species with direct mutagenic effects to deregulate the immune system and promote angiogenesis, which is essential for the neovascularization and survival of tumors.
Individuals infected with HIV-1 have a high risk of cancers caused by another infectious agent. HIV-1 infection, mainly through immunosuppression, leads to increased replication of oncogenic viruses such as EBV and KSHV. Although antiretroviral therapy lowers the risk of many cancers associated with HIV-1, risks remain high worldwide.
5 Lifetime Cumulative Incidence of Some Virus-caused Cancers
Some viruses may cause more than one cancer, while some cancers may be caused by more than one virus. However, only a proportion of persons infected by these oncogenic viruses will develop specific cancers. Table 3 shows the lifetime cumulative incidence of some virus-caused cancers. The cumulative lifetime (30–75 years old) risk of developing nasopharyngeal carcinoma was 2.2 % for men seropositive for IgA antibodies against EBV VCA or antibodies against EBV DNase and 0.48 % for those seronegative for both antibodies.
Table 3
Lifetime cumulative incidence and risk cofactors of virus-caused cancers
Virus (cancer) | Lifetime incidence | Viral factors | Host factors | Environmental factor |
|---|---|---|---|---|
EBV (nasopharyngeal carcinoma) | Men, 2.0 %. | Elevated serotiter of antibodies against EBV, EBV viral load | Male gender, family history, genetic polymorphisms (xenobiotic metabolism, DNA repair, human leukocyte antigen) | Cantonese salted fish, Dietary nitrosamine, wood dust, formaldehyde, tobacco |
HBV (hepatocellular carcinoma) | Men, 27.4 %; women, 8.0 %. | Persistent infection, viral load, genotype, mutant, serum HBsAg level | Elder age, male gender, obesity, diabetes, serum androgen and ALT level, family history, genetic polymorphisms (DNA repair, human leukocyte antigen, androgen, and xenobiotic metabolism) | Aflatoxins, alcohol, tobacco, carotenoids, selenium, HCV infection |
HCV (hepatocellular carcinoma) | Men, 23.7 %; women, 16.7 % | Persistent infection, viral load, genotype, mutant | Elder age, male gender, obesity, diabetes, serum ALT level, family history, genetic polymorphisms | Alcohol, tobacco, betel, HBV or HTLV-1 infection, radiation |
HPV (cervical carcinoma) | HPV-16, 34.3 %; HPV-52, 23.3 %; HPV-58, 33.4 %; Any oncogenic HPV, 20.3 %. | Persistent infection, viral load, genotype
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|